Synergistic Anti-Angiogenic Effects Using Peptide-Based Combinatorial Delivery of siRNAs Targeting VEGFA, VEGFR1, and Endoglin Genes
Abstract
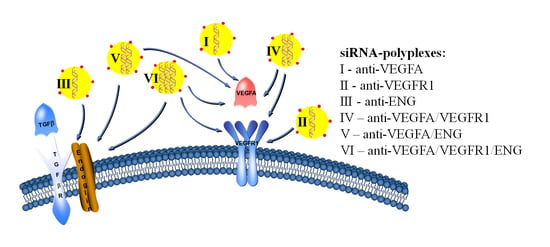
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Peptide Synthesis and Design
2.3. siRNA Preparation of Peptide/siRNA Complexes
2.4. Cytotoxicity Assay
2.5. siRNA Transfer to MDA-MB-231 Cells
2.6. siRNA Transfer to ЕА.Hy926 Cells
2.7. Quantitative RT-PCR
2.8. Scratch Migration Assay
2.9. Proliferation Assays
2.10. Statistical Analysis
3. Results and Discussion
3.1. Cytotoxicity Evaluation of L1 Peptide/siRNA Complexes
3.2. In Vitro Transfection of MDA-MB-231 Cells
3.3. In Vitro Transfection of ЕА.Hy926 Cells
3.4. Inhibition of the Endothelial Cells Migration
3.5. Inhibition of the Endothelial Cells Proliferation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, C.; Kim, T.M.; Malik, A.B. Transcriptional regulation of endothelial cell and vascular development. Circ. Res. 2013, 112, 1380–1400. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Gacche, R.N.; Meshram, R.J. Angiogenic factors as potential drug target: Efficacy and limitations of anti-angiogenic therapy. Biochim. Et Biophys. Acta—Rev. Cancer 2014, 1846, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.; Folkman, J. Clinical translation of angiogenesis inhibitors. Nat. Rev. Cancer 2002, 2, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Shubina, A.N.; Egorova, A.A.; Baranov, V.S.; Kiselev, A.V. Recent advances in gene therapy of endometriosis. Recent Pat. Dna Gene Seq. 2013, 7, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Kang, G.; Wang, T.; Huang, H.E. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol. Lett. 2018, 16, 687–702. [Google Scholar] [CrossRef]
- Ferrara, N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am.J.Physiol.—CellPhysiol. 2001, 280, C1358–C1366. [Google Scholar] [CrossRef]
- Behrouz, R.; Malek, A.R.; Torbey, M.T. Small vessel cerebrovascular disease: The past, present, and future. StrokeRes.Treat. 2012, 2012, 839151. [Google Scholar] [CrossRef]
- Gaengel, K.; Genové, G.; Armulik, A.; Betsholtz, C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Boil. 2009, 29, 630–638. [Google Scholar] [CrossRef]
- Liu, S.; Kong, X.; Ge, D.; Wang, S.; Zhao, J.; Su, L.; Zhang, S.; Zhao, B.; Miao, J. Identification of new small molecules as apoptosis inhibitors in vascular endothelial cells. J. Cardiovasc. Pharmacol. 2016, 67, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.P.; Hillan, K.J.; Ryan, A.M.; Kowalski, J.; Keller, G.A.; Rangell, L.; Wright, B.D.; Radtke, F.; Aguet, M.; Ferrara, N. VEGF is required for growth and survival in neonatal mice. Development 1999, 126, 1149–1159. [Google Scholar] [PubMed]
- Safran, M.; Kaelin, W.G., Jr. HIF hydroxylation and the mammalian oxygen-sensing pathway. J. Clin. Investig. 2003, 111, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Morfoisse, F.; Renaud, E.; Hantelys, F.; Prats, A.-C.; Garmy-Susini, B. Role of hypoxia and vascular endothelial growth factors in lymphangiogenesis. Mol.Cell.Oncol. 2015, 2, e1024821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.; Li, B.; Li, Z.; Cutler, S.J.; Rankin, G.O.; Chen, Y.C. Chaetoglobosin K inhibits tumor angiogenesis through downregulation of vascular epithelial growth factor-binding hypoxia-inducible factor 1α. Anti-Cancer Drugs 2013, 24, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Mazzone, M.; Jonckx, B.; Carmeliet, P. FLT1 and its ligands VEGFB and PlGF: Drug targets for anti-angiogenic therapy? Nat. Rev. Cancer 2008, 8, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Kami, J.; Muranaka, K.; Yanagi, Y.; Obata, R.; Tamaki, Y.; Shibuya, M. Inhibition of choroidal neovascularization by blocking vascular endothelial growth factor receptor tyrosine kinase. Jpn. J. Ophthalmol. 2008, 52, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Golfmann, K.; Meder, L.; Koker, M.; Volz, C.; Borchmann, S.; Tharun, L.; Dietlein, F.; Malchers, F.; Florin, A.; Büttner, R.; et al. Synergistic anti-angiogenic treatment effects by dual FGFR1 and VEGFR1 inhibition in FGFR1-amplified breast cancer. Oncogene 2018, 37, 5682–5693. [Google Scholar] [CrossRef]
- Cao, Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci.Signal. 2009, 2, re1. [Google Scholar] [CrossRef]
- Derbyshire, E.J.; Gazdar, A.F.; King, S.W.; Thorpe, P.E.; Derbyshire, E.J.; King, S.W.; Thorpe, P.E.; Gazdar, A.F.; Vitetta, E.S.; Tazzari, P.L.; et al. Up-Regulation of Endoglin on Vascular Endothelial Cells in Human Solid Tumors: Implications for Diagnosis and Therapy. Clin. Cancer Res. 1995, 1, 1623–1634. [Google Scholar]
- Nassiri, F.; Cusimano, M.D.; Scheithauer, B.W.; Rotondo, F.; Fazio, A.; Yousef, G.M.; Syro, L.V.; Kovacs, K.; Lloyd, R.V. Endoglin (CD105): A review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011, 31, 2283–2290. [Google Scholar] [PubMed]
- Li, D.Y.; Sorensen, L.K.; Brooke, B.S.; Urness, L.D.; Davis, E.C.; Taylor, D.G.; Boak, B.B.; Wendel, D.P. Defective angiogenesis in mice lacking endoglin. Science 1999, 284, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Taskiran, C.; Erdem, O.; Onan, A.; Arisoy, O.; Acar, A.; Vural, C.; Erdem, M.; Ataoglu, O.; Guner, H. The prognostic value of endoglin (CD105) expression in ovarian carcinoma. Int. J. Gynecol. Cancer 2006, 16, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A.; Adamek, A. Role of Endoglin (CD105) in the Progression of Hepatocellular Carcinoma and Anti-Angiogenic Therapy. Int. J. Mol. Sci. 2018, 19, 3887. [Google Scholar] [CrossRef] [PubMed]
- Seon, B.K.; Haba, A.; Matsuno, F.; Norihiko Takahashi, M.T.; She, X.; Harada, N.; Uneda, S.; Tsujie, T.; Toi, H.; Hilda Tsai, Y.H. Endoglin-targeted cancer therapy. Curr. Drug Deliv. 2011, 8, 135–143. [Google Scholar] [CrossRef]
- Smirnov, I.V.; Gryazeva, I.V.; Samoilovich, M.P.; Klimovich, V.B. Endoglin (CD105) - A target for visualization and anti-angiogenic therapy for malignant tumors. Vopr. Onkol. 2015, 61, 898–907. [Google Scholar]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- David, S.; Pitard, B.; Benoît, J.P.; Passirani, C. Non-viral nanosystems for systemic siRNA delivery. Pharm. Res. 2010, 62, 100–114. [Google Scholar] [CrossRef] [Green Version]
- Le Bon, B.; Van Craynest, N.; Daoudi, J.M.; Di Giorgio, C.; Domb, A.J.; Vierling, P. AMD3100 Conjugates as Components of Targeted Nonviral Gene Delivery Systems: Synthesis and in Vitro Transfection Efficiency of CXCR4-Expressing Cells. Bioconjug. Chem. 2004, 15, 413–423. [Google Scholar] [CrossRef]
- Driessen, W.H.P.; Fujii, N.; Tamamura, H.; Sullivan, S.M. Development of peptide-targeted lipoplexes to CXCR4-expressing rat glioma cells and rat proliferating endothelial cells. Mol. Ther. 2008, 16, 516–524. [Google Scholar] [CrossRef]
- Egorova, A.; Kiselev, A.; Hakli, M.; Ruponen, M.; Baranov, V.; Urtti, A. Chemokine-derived peptides as carriers for gene delivery to CXCR4 expressing cells. J. Gene Med. 2009, 11, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Y.; Oupický, D. Potential of CXCR4 / CXCL12 Chemokine Axis in Cancer Drug Delivery. Curr. Pharmacol. Rep. 2016, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Juarez, J.; Bendall, L.; Bradstock, K. Chemokines and their Receptors as Therapeutic Targets: The Role of the SDF-1 / CXCR4 Axis. Curr. Pharm. Des. 2005, 10, 1245–1259. [Google Scholar] [CrossRef]
- Salcedo, R.; Oppenheim, J.J. Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation 2003, 10, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, A.; Egorova, A.; Laukkanen, A.; Baranov, V.; Urtti, A. Characterization of reducible peptide oligomers as carriers for gene delivery. Int. J. Pharm. 2013, 441, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Egorova, A.; Bogacheva, M.; Shubina, A.; Baranov, V.; Kiselev, A. Development of a receptor-targeted gene delivery system using CXCR4 ligand-conjugated cross-linking peptides. J.GeneMed. 2014, 16, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Egorova, A.; Shubina, A.; Sokolov, D.; Selkov, S.; Baranov, V.; Kiselev, A. CXCR4-targeted modular peptide carriers for efficient anti-VEGF siRNA delivery. Int.J.Pharm. 2016, 515, 431–440. [Google Scholar] [CrossRef]
- Egorova, A.; Petrosyan, M.; Maretina, M.; Balashova, N.; Polyanskih, L.; Baranov, V.; Kiselev, A. Anti-angiogenic treatment of endometriosis via anti-VEGFA siRNA delivery by means of peptide-based carrier in a rat subcutaneous model. Gene Ther. 2018, 25, 548–555. [Google Scholar] [CrossRef]
- Egorova, A.A.; Maretina, M.A.; Kiselev, A.V. VEGFA Gene Silencing in CXCR4-Expressing Cells via siRNA Delivery by Means of Targeted Peptide Carrier. Methods Mol. Biol. 2019, 1974, 57–68. [Google Scholar]
- Slita, A.; Egorova, A.; Casals, E.; Kiselev, A.; Rosenholm, J.M. Characterization of modified mesoporous silica nanoparticles as vectors for siRNA delivery. Asian J. Pharm. Sci. 2018, 13, 592–599. [Google Scholar] [CrossRef]
- Edgell, C.J.S.; McDonald, C.C.; Graham, J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA 1983, 80, 3734–3737. [Google Scholar] [CrossRef] [PubMed]
- De Fougerolles, A.; Frank-Kamenetsky, M.; Manoharan, M.; Rajeev, K.G.; Hadwiger, P. IRNA Agents Targeting VEGF, U.S. Patent No. 7,919,473; U.S. Patent and Trademark Office: Washington, DC, USA, 2011.
- Zhou, Z.; Zhao, C.; Wang, L.; Cao, X.; Li, J.; Huang, R.; Lao, Q.; Yu, H.; Li, Y.; Du, H.; et al. A VEGFR1 antagonistic peptide inhibits tumor growth and metastasis through VEGFR1-PI3K-AKT signaling pathway inhibition. Am. J. Cancer Res. 2015, 5, 3149–3161. [Google Scholar] [PubMed]
- Dolinsek, T.; Markelc, B.; Bosnjak, M.; Blagus, T.; Prosen, L.; Kranjc, S.; Stimac, M.; Lampreht, U.; Sersa, G.; Cemazar, M. Endoglin silencing has significant antitumor effect on murine mammary adenocarcinoma mediated by vascular targeted effect. Curr.GeneTher. 2015, 15, 228–244. [Google Scholar] [CrossRef]
- Raemdonck, K.; Naeye, B.; Høgset, A.; Demeester, J.; De Smedt, S.C. Prolonged gene silencing by combining siRNA nanogels and photochemical internalization. J.Control.Release 2010, 145, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Qu, L.; Meng, L.; Zeng, Y.; Shou, C.; Xu, H.; Jiang, B.; Ren, T. N -α-Acetyltransferase 10 protein inhibits apoptosis through RelA/p65-regulated MCL1 expression. Carcinogenesis 2012, 33, 1193–1202. [Google Scholar]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: advances in siRNA delivery. Nat.Rev.Drug Discov. 2009, 8, 129. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar]
- Wang, Z.; Ma, Y.; Yu, X.; Niu, Q.; Han, Z.; Wang, H.; Li, T. Targeting CXCR4 – CXCL12 Axis for Visualizing, Predicting, and Inhibiting Breast Cancer Metastasis with Theranostic AMD3100 – Ag2S Quantum Dot Probe. Adv. Funct. Mater. 2018, 28, 1800732. [Google Scholar] [CrossRef]
- Vega-Villa, K.R.; Takemoto, J.K.; Yáñez, J.A.; Remsberg, C.M.; Forrest, M.L.; Davies, N.M. Clinical toxicities of nanocarrier systems. Adv. Drug Deliv. Rev. 2008, 60, 929–938. [Google Scholar] [CrossRef]
- Vaidyanathan, S.; Anderson, K.B.; Merzel, R.L.; Jacobovitz, B.; Kaushik, M.P.; Kelly, C.N.; Van Dongen, M.A.; Dougherty, C.A.; Orr, B.G.; Banaszak Holl, M.M. Quantitative Measurement of Cationic Polymer Vector and Polymer-pDNA Polyplex Intercalation into the Cell Plasma Membrane. ACS Nano 2015, 9, 6097–6109. [Google Scholar] [CrossRef]
- Jones, N.A.; Hill, I.R.C.; Stolnik, S.; Bignotti, F.; Davis, S.S.; Garnett, M.C. Polymer chemical structure is a key determinant of physicochemical and colloidal properties of polymer–DNA complexes for gene delivery. Biochim. Biophys. Acta—Gene Struct. Expr. 2000, 1517, 1–18. [Google Scholar] [CrossRef]
- Herbert, S.P.; Stainier, D.Y.R. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 551. [Google Scholar] [CrossRef] [PubMed]
- Herzog, B.; Pellet-Many, C.; Britton, G.; Hartzoulakis, B.; Zachary, I.C. VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol. Boil. Cell 2011, 22, 2766–2776. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.; Oda, N.; Abe, M.; Terai, Y.; Ito, M.; Shitara, K.; Tabayashi, K.; Shibuya, M.; Sato, Y. Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene 2000, 19, 2138–2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warrington, K.; Hillarby, M.C.; Li, C.; Letarte, M.; Kumar, S. Functional role of CD105 in TGF-β1 signalling in murine and human endothelial cells. Anticancer Res. 2005, 25, 1851–1864. [Google Scholar]
- Lee, N.Y.; Ray, B.; How, T.; Blobe, G.C. Endoglin promotes transforming growth factor β-mediated Smad 1/5/8 signaling and inhibits endothelial cell migration through its association with GIPC. J. Biol. Chem. 2008, 283, 32527–32533. [Google Scholar] [CrossRef] [PubMed]
- Castanotto, D.; Sakurai, K.; Lingeman, R.; Li, H.; Shively, L.; Aagaard, L.; Soifer, H.; Gatignol, A.; Riggs, A.; Rossi, J.J. Combinatorial delivery of small interfering RNAs reduces RNAi efficacy by selective incorporation into RISC. Nucleic Acids Res. 2007, 35, 5154–5164. [Google Scholar] [CrossRef]
- Tiash, S.; Kamaruzman, N.I.B.; Chowdhury, E.H. Carbonate apatite nanoparticles carry siRNA(S) targeting growth factor receptor genes egfr1 and erbb2 to regress mouse breast tumor. Drug Deliv. 2017, 24, 1721–1730. [Google Scholar] [CrossRef]
- Michiue, H.; Eguchi, A.; Scadeng, M.; Dowdy, S.F. Induction of in vivo synthetic lethal RNAi responses to treat glioblastoma. Cancer Biol. 2009, 8, 2306–2313. [Google Scholar] [CrossRef]
- Kamaruzman, N.; Tiash, S.; Ashaie, M.; Chowdhury, E. siRNAs Targeting Growth Factor Receptor and Anti-Apoptotic Genes Synergistically Kill Breast Cancer Cells through Inhibition of MAPK and PI-3 Kinase Pathways. Biomedicines 2018, 6, 73. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, P.; Mayhew, T.M.; Charnock-Jones, D.S. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta 2004, 25, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Seetharam, L.; Gotoh, N.; Maru, Y.; Neufeld, G.; Yamaguchi, S.; Shibuya, M. A unique signal transduction from FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene 1995, 10, 135–147. [Google Scholar] [PubMed]
- Pan, C.C.; Bloodworth, J.C.; Mythreye, K.; Lee, N.Y. Endoglin inhibits ERK-induced c-Myc and cyclin D1 expression to impede endothelial cell proliferation. Biochem. Biophys. Res. Commun. 2012, 424, 620–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Yook, S.; Yhee, J.Y.; Yoon, H.Y.; Kim, M.G.; Ku, S.H.; Kim, S.H.; Park, J.H.; Jeong, J.H.; Kwon, I.C.; et al. Co-delivery of VEGF and Bcl-2 dual-targeted siRNA polymer using a single nanoparticle for synergistic anti-cancer effects in vivo. J. Control. Release 2015, 220, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Han, H.D.; Ahn, H.J. A RNA nanotechnology platform for a simultaneous two-in-one siRNA delivery and its application in synergistic RNAi therapy. Sci. Rep. 2016, 6, 32363. [Google Scholar] [CrossRef]
- Hu, J.; Guan, W.; Liu, P.; Dai, J.; Tang, K.; Xiao, H.; Qian, Y.; Sharrow, A.C.; Ye, Z.; Wu, L.; et al. Endoglin Is Essential for the Maintenance of Self-Renewal and Chemoresistance in Renal Cancer Stem Cells. Stem Cell Rep. 2017, 9, 464–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Gómez, E.; Eleno, N.; López-Novoa, J.M.; Ramirez, J.R.; Velasco, B.; Letarte, M.; Bernabéu, C.; Quintanilla, M. Characterization of murine S-endoglin isoform and its effects on tumor development. Oncogene 2005, 24, 4450–4461. [Google Scholar] [Green Version]
- Clarke, J.M.; Hurwitz, H.I. Understanding and targeting resistance to anti-angiogenic therapies. J. Gastrointest. Oncol. 2013, 4, 253–263. [Google Scholar]








| Type of Analysis | Type of siRNA | |||||
|---|---|---|---|---|---|---|
| Anti-VEGFA | Anti-VEGFR1 | Anti-Endoglin | Anti-VEGFA + Anti-VEGFR1 | Anti-VEGFA + Anti-Endoglin | Anti-VEGFA + Anti-VEGFR1 + Anti-Endoglin | |
| Migration | ↓ | ↓ | ↓ | ↓ | ↓↓ | NA |
| Proliferation | ↓ | − | ↓/− | ↓↓ | − | − |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorova, A.A.; Shtykalova, S.V.; Maretina, M.A.; Sokolov, D.I.; Selkov, S.A.; Baranov, V.S.; Kiselev, A.V. Synergistic Anti-Angiogenic Effects Using Peptide-Based Combinatorial Delivery of siRNAs Targeting VEGFA, VEGFR1, and Endoglin Genes. Pharmaceutics 2019, 11, 261. https://doi.org/10.3390/pharmaceutics11060261
Egorova AA, Shtykalova SV, Maretina MA, Sokolov DI, Selkov SA, Baranov VS, Kiselev AV. Synergistic Anti-Angiogenic Effects Using Peptide-Based Combinatorial Delivery of siRNAs Targeting VEGFA, VEGFR1, and Endoglin Genes. Pharmaceutics. 2019; 11(6):261. https://doi.org/10.3390/pharmaceutics11060261
Chicago/Turabian StyleEgorova, Anna A., Sofia V. Shtykalova, Marianna A. Maretina, Dmitry I. Sokolov, Sergei A. Selkov, Vladislav S. Baranov, and Anton V. Kiselev. 2019. "Synergistic Anti-Angiogenic Effects Using Peptide-Based Combinatorial Delivery of siRNAs Targeting VEGFA, VEGFR1, and Endoglin Genes" Pharmaceutics 11, no. 6: 261. https://doi.org/10.3390/pharmaceutics11060261
APA StyleEgorova, A. A., Shtykalova, S. V., Maretina, M. A., Sokolov, D. I., Selkov, S. A., Baranov, V. S., & Kiselev, A. V. (2019). Synergistic Anti-Angiogenic Effects Using Peptide-Based Combinatorial Delivery of siRNAs Targeting VEGFA, VEGFR1, and Endoglin Genes. Pharmaceutics, 11(6), 261. https://doi.org/10.3390/pharmaceutics11060261