Enhancement of Magnetic Hyperthermia by Mixing Synthetic Inorganic and Biomimetic Magnetic Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. BMNPs and MNPs Production
2.2. Nanoparticle Characterization
3. Results and Discussion
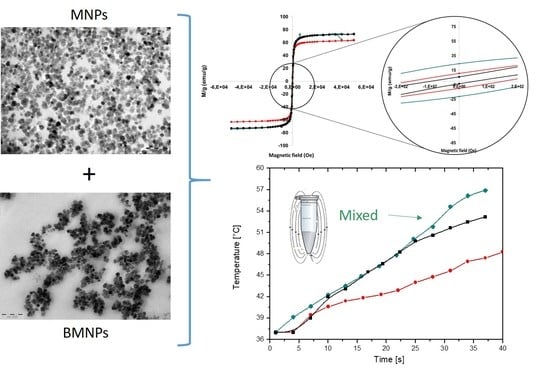
3.1. Particle Characterization
3.2. Zeta Potential
3.3. Stability
3.4. Performance in Hyperthermia
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rezayan, A.H.; Mousavi, M.; Kheirjou, S.; Amoabediny, G.; Ardestani, M.S.; Mohammadnejad, J. Monodisperse magnetite (Fe3O4) nanoparticles modified with water soluble polymers for the diagnosis of breast cancer by MRI method. J. Magn. Magn. Mater. 2016, 420, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Osorio, Z.; Argibay, B.; Pineiro, Y.; Vazquez-Vazquez, C.; Lopez-Quintela, M.A.; Alvarez-Perez, M.A.; Sobrino, T.; Campos, F.; Castillo, J.; Rivas, J. Multicore magnetic Fe3O4@C beads with enhanced magnetic response for MRI in brain biomedical applications. IEEE Trans. Magn. 2016, 52, 1–4. [Google Scholar] [CrossRef]
- Portillo, M.A.; Iglesias, G.R. Magnetic nanoparticles as a redispersing additive in magnetorheological fluid. J. Nanomater. 2017, 2017, 8. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Banobre-Lopez, M.; Martins, P.; Lanceros-Mendez, S. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef]
- Ghazi, N.; Chenari, H.M.; Ghodsi, F.E. Rietveld refinement, morphology analysis, optical and magnetic properties of magnesium-zinc ferrite nanofibers. J. Magn. Magn. Mater. 2018, 468, 132–140. [Google Scholar] [CrossRef]
- Lisjak, D.; Mertelj, A. Anisotropic magnetic nanoparticles: A review of their properties, syntheses and potential applications. Prog. Mater. Sci. 2018, 95, 286–328. [Google Scholar] [CrossRef]
- Muhlberger, M.; Janko, C.; Unterweger, H.; Schreiber, E.; Band, J.; Lehmann, C.; Dudziak, D.; Lee, G.; Alexiou, C.; Tietze, R. Functionalization of T lymphocytes for magnetically controlled immune therapy: Selection of suitable superparamagnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2019, 473, 61–67. [Google Scholar] [CrossRef]
- Danilushkina, A.; Rozhina, E.; Kamalieva, R.; Fakhrullin, R. Influence of magnetic nanoparticles stabilized with polyelectrolytes on 2D and 3D cell cultures formation. Hum. Gene Ther. 2018, 29, A71. [Google Scholar]
- Chen, K.L.; Yeh, Y.W.; Chen, J.M.; Hong, Y.J.; Huang, T.L.; Deng, Z.Y.; Wu, C.H.; Liao, S.H.; Wang, L.M. Influence of magnetoplasmonic gamma-Fe2O3/Au core/shell nanoparticles on low-field nuclear magnetic resonance. Sci. Rep. 2016, 6, 35477. [Google Scholar] [CrossRef]
- Wang, W.; Ma, P.X.; Dong, H.; Krause, H.J.; Zhang, Y.; Willbold, D.; Offenhaeusser, A.; Gu, Z.W. A magnetic nanoparticles relaxation sensor for protein-protein interaction detection at ultra-low magnetic field. Biosens. Bioelectron. 2016, 80, 661–665. [Google Scholar] [CrossRef]
- Angelakeris, M. Magnetic nanoparticles: A multifunctional vehicle for modern theranostics. Biochim. Biophys. Acta Gen. Subj. 2018, 1861, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Engels, S.; Reinhardt, L.; Wasylow, C.; Gerullis, H.; Wawroschek, F. Magnetic marking and intraoperative detection of primary draining lymph nodes in high-risk prostate cancer using superparamagnetic iron oxide nanoparticles: Additional diagnostic value. Molecules 2017, 22, 2192. [Google Scholar] [CrossRef] [PubMed]
- Duran, J.D.G.; Arias, J.L.; Gallardo, V.; Delgado, A.V. Magnetic colloids as drug vehicles. J. Pharm. Sci. 2008, 97, 2948–2983. [Google Scholar] [CrossRef] [PubMed]
- Patitsa, M.; Karathanou, K.; Kanaki, Z.; Tzioga, L.; Pippa, N.; Demetzos, C.; Verganelakis, D.A.; Cournia, Z.; Klinakis, A. Magnetic nanoparticles coated with polyarabic acid demonstrate enhanced drug delivery and imaging properties for cancer theranostic applications. Sci. Rep. 2017, 7, 775. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Xie, W.S.; Wang, Y.; Wang, D.; Guo, Z.H.; Gao, F.; Zhao, L.Y.; Cai, Q. A theranostic nanocomposite system based on radial mesoporous silica hybridized with Fe3O4 nanoparticles for targeted magnetic field responsive chemotherapy of breast cancer. Rsc Adv. 2018, 8, 4321–4328. [Google Scholar] [CrossRef]
- Moskvin, M.; Babic, M.; Reis, S.; Cruz, M.M.; Ferreira, L.P.; Carvalho, M.D.; Lima, S.A.C.; Horák, D. Biological evaluation of surface-modified magnetic nanoparticles as a platform for colon cancer cell theranostics. Colloids Surf. B Biointerfaces 2018, 161, 35–41. [Google Scholar] [CrossRef]
- Semkina, A.S.; Abakumov, M.A.; Skorikov, A.S.; Abakumova, T.O.; Melnikov, P.A.; Grinenko, N.F.; Cherepanov, S.A.; Vishnevskiy, D.A.; Naumenko, V.A.; Ionova, K.P.; et al. Multimodal doxorubicin loaded magnetic nanoparticles for VEGF targeted theranostics of breast cancer. Nanomed. Nanoechnol. 2018, 14, 1733–1742. [Google Scholar] [CrossRef]
- Vakilinezhad, M.A.; Alipour, S.; Montaseri, H. Fabrication and in vitro evaluation of magnetic PLGA nanoparticles as a potential Methotrexate delivery system for breast cancer. J. Drug Deliv. Sci. Technol. 2018, 44, 467–474. [Google Scholar] [CrossRef]
- Obaidat, L.M.; Issa, B.; Haik, Y. Magnetic properties of magnetic nanoparticles for efficient hyperthermia. Nanomaterials (Basel) 2015, 5, 63–89. [Google Scholar] [CrossRef]
- Balidemaj, E.; Kok, H.P.; Schooneveldt, G.; van Lier, A.; Remis, R.F.; Stalpers, L.J.A.; Westerveld, H.; Nederveen, A.J.; van den Berg, C.A.T.; Crezee, J. Hyperthermia treatment planning for cervical cancer patients based on electrical conductivity tissue properties acquired in vivo with EPT at 3 T MRI. Int. J. Hyperth. 2016, 32, 558–568. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine (Lond) 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, G.; Delgado, A.V.; Kujda, M.; Ramos-Tejada, M.M. Magnetic hyperthermia with magnetite nanoparticles: Electrostatic and polymeric stabilization. Colloid Polym. Sci. 2016, 294, 1541–1550. [Google Scholar] [CrossRef]
- Sato, L.; Umemura, M.; Mitsudo, K.; Fukumura, H.; Kim, J.H.; Hoshino, Y.; Nakashima, H.; Kioi, M.; Nakakaji, R.; Sato, M.; et al. Simultaneous hyperthermia-chemotherapy with controlled drug delivery using single-drug nanoparticles. Sci. Rep. 2016, 6, 24629. [Google Scholar] [CrossRef] [PubMed]
- Hedayatnasab, Z.; Abnisa, F.; Daud, W.M.A.W. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017, 123, 174–196. [Google Scholar] [CrossRef]
- Nemati, Z.; Salili, S.M.; Alonso, J.; Ataie, A.; Das, R.; Phan, M.H.; Srikanth, H. Superparamagnetic iron oxide nanodiscs for hyperthermia therapy: Does size matter? J. Alloy. Compd. 2017, 714, 709–714. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Delgado, A.V.; Schneider, E.K.; Fernandez, B.C.L.; Iglesias, G.R. Magnetic nanoparticles coated with a thermosensitive polymer with hyperthermia properties. Polymers (Basel) 2018, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Vamvakidis, K.; Mourdikoudis, S.; Makridis, A.; Paulidou, E.; Angelakeris, M.; Dendrinou-Samara, C. Magnetic hyperthermia efficiency and MRI contrast sensitivity of colloidal soft/hard ferrite nanoclusters. J. Colloid Interface Sci. 2018, 511, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D 2003, 36, R167–R181. [Google Scholar] [CrossRef] [Green Version]
- Soares, P.I.P.; Ferreira, L.M.M.; Igreja, R.; Novo, C.M.M.; Borges, J. Application of hyperthermia for cancer treatment: Recent patents review. Recent Pat. Anticancer Drug Discov. 2012, 7, 64–73. [Google Scholar] [CrossRef]
- Alphandery, E.; Faure, S.; Seksek, O.; Guyot, F.; Chebbi, I. Chains of magnetosomes extracted from AMB-1 magnetotactic bacteria for application in alternative magnetic field cancer therapy. Acs Nano 2011, 5, 6279–6296. [Google Scholar] [CrossRef]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Preparation, conjugation and delivery. Nanomedicine (Lond) 2018, 13, 929–952. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Tercedor, C.; Montalban-Lopez, M.; Perez-Gonzalez, T.; Sanchez-Quesada, M.S.; Prozorov, T.; Pineda-Molina, E.; Fernandez-Vivas, M.A.; Rodriguez-Navarro, A.B.; Trubitsyn, D.; Bazylinski, D.A.; et al. Size control of in vitro synthesized magnetite crystals by the MamC protein of Magnetococcus marinus strain MC-1. Appl. Microbiol. Biotechnol. 2015, 99, 5109–5121. [Google Scholar] [CrossRef] [PubMed]
- García Rubia, G.; Peigneux, A.; Jabalera, Y.; Puerma, J.; Oltolina, F.; Elert, K.; Colangelo, D.; Morales, J.G.; Prat, M.; Jimenez-Lopez, C. pH-dependent adsorption release of doxorubicin on MamC-biomimetic magnetite nanoparticles. Langmuir 2018, 34, 13713–13724. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, L.M.; Malaki, R.B.; Carrey, J.; Lachaize, S.; Respaud, M.; Goya, G.F.; Chaudret, B. Magnetic hyperthermia in single-domain monodisperse FeCo nanoparticles: Evidences for Stoner-Wohlfarth behavior and large losses. J. Appl. Phys. 2009, 105, 023911. [Google Scholar] [CrossRef]
- Carrey, J.; Mehdaoui, B.; Respaud, M. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: Application to magnetic hyperthermia optimization. J. Appl. Phys. 2011, 110, 083921. [Google Scholar] [CrossRef]
- Mehdaoui, B.; Meffre, A.; Carrey, J.; Lachaize, S.; Lacroix, L.M.; Gougeon, M.; Chaudret, B.; Respaud, M. Optimal size of nanoparticles for magnetic hyperthermia: A combined theoretical and experimental study. Adv. Funct. Mater. 2011, 21, 4573–4581. [Google Scholar] [CrossRef]
- Stoner, E.C.; Wohlfarth, E.P. A mechanism of magnetic hysteresis in heterogeneous alloys. IEEE Trans. Magn. 1991, 27, 3475–3518. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001. [Google Scholar] [CrossRef]
- Ortega, D.; Pankhurst, Q.A. Magnetic hyperthermia. In Nanoscience: Nanostructures through Chemistry; O’Brien, P., Ed.; Royal Society of Chemistry: Cambridge, UK, 2013; Volume 1, pp. 60–88. [Google Scholar]
- Surowiec, Z.; Miaskowski, A.; Budzynski, M. Investigation of magnetite Fe3O4 nanoparticles for magnetic hyperthermia. Nukleonika 2017, 62, 183–186. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Hafeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Coffey, W.T.; Crothers, D.S.F.; Kalmykov, Y.P.; Waldron, J.T. Constant-magnetic-field effect in Néel relaxation of single-domain ferromagnetic particles. Phys. Rev. B Condens. Matter. 1995, 51, 15947–15956. [Google Scholar] [CrossRef] [PubMed]
- Hergt, R.; Dutz, S.; Zeisberger, M. Validity limits of the Neel relaxation model of magnetic nanoparticles for hyperthermia. Nanotechnology 2010, 21, 015706. [Google Scholar] [CrossRef] [PubMed]
- Bakoglidis, K.D.; Simeonidis, K.; Sakellari, D.; Stefanou, G.; Angelakeris, M. Size-Dependent mechanisms in AC magnetic hyperthermia response of iron-oxide nanoparticles. IEEE Trans. Magn. 2012, 48, 1320–1323. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia-a promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef] [PubMed]
- Guibert, C.; Dupuis, V.; Peyre, V.; Fresnais, J. Hyperthermia of magnetic nanoparticles: Experimental study of the role of aggregation. J. Phys. Chem. C 2015, 119, 28148–28154. [Google Scholar] [CrossRef]
- Fortin, J.P.; Wilhelm, C.; Servais, J.; Menager, C.; Bacri, J.C.; Gazeau, F. Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J. Am. Chem. Soc. 2007, 129, 2628–2635. [Google Scholar] [CrossRef]
- Perez-Gonzalez, T.; Rodriguez-Navarro, A.; Jimenez-Lopez, C. Inorganic magnetite precipitation at 25 °C: A low-cost inorganic coprecipitation method. J. Supercond. Nov. Magn. 2011, 24, 549–557. [Google Scholar] [CrossRef]
- Gas, P.; Kurgan, E. Cooling effects inside water-cooled inductors for magnetic fluid hyperthermia. In Proceedings of the 2017 Progress in Applied Electrical Engineering (PAEE), Koscielisko, Poland, 25–30 June 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Gas, P.; Miaskowski, A. Specifying the ferrofluid parameters important from the viewpoint of Magnetic Fluid Hyperthermia. In Proceedings of the 2015 Selected Problems of Electrical Engineering and Electronics (WZEE), Kielce, Poland, 17–19 September 2015; pp. 1–6. [Google Scholar] [CrossRef]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Muniz, F.T.L.; Miranda, M.A.R.; dos Santos, C.M.; Sasaki, J.M. The Scherrer equation and the dynamical theory of X-ray diffraction. Acta Crystallogr. A Found. Adv. 2016, A72, 385–390. [Google Scholar] [CrossRef]
- Lopez-Moreno, R.; Fernandez-Vivas, A.; Valverde-Tercedor, C.; Azuaga Fortes, A.I.; Casares Atienza, S.; Rodriguez-Navarro, A.B.; Zarivach, R.; Jimenez-Lopez, C. Magnetite nanoparticles biomineralization in the presence of the magnetosome membrane protein MamC: Effect of protein aggregation and protein atructure on magnetite formation. Cryst. Growth Des. 2017, 17, 1620–1629. [Google Scholar] [CrossRef]
- Fonseca, F.C.; Goya, G.F.; Jardim, R.F.; Muccillo, R.; Carreno, N.L.V.; Longo, E.; Leite, E.R. Superparamagnetism and magnetic properties of Ni nanoparticles embedded in SiO2. Phys. Rev. B 2002, 66, 104406. [Google Scholar] [CrossRef]
- Obrien, R.W.; White, L.R. Electrophoretic mobility of a spherical colloidal particle. J. Chem. Soc. Faraday Trans. 2 1978, 74, 1607–1626. [Google Scholar] [CrossRef]
- Nudelman, H.; Valverde-Tercedor, C.; Kolusheva, S.; Perez-Gonzalez, T.; Widdrat, M.; Grimberg, N.; Levi, H.; Nelkenbaum, O.; Davidov, G.; Faivre, D.; et al. Structure-function studies of the magnetite-biomineralizing magnetosome-associated protein MamC. J. Struct. Biol. 2016, 194, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Jabalera, Y.; Casares Atienza, S.; Fernandez-Vivas, A.; Peigneux, A.; Azuaga Fortes, A.I.; Jimenez-Lopez, C. Protein conservation method affects MamC-mediated biomineralization of magnetic nanoparticles. Cryst. Growth Des. 2019, 19, 1064–1071. [Google Scholar] [CrossRef]








| System | Frequency f [kHz] | SAR [W/g] | ILP [nHm2kg−1] | ΔT [°C] |
|---|---|---|---|---|
| MNPs | 197 | 64 ± 5 | 1.01 ± 0.07 | 20.2 ± 0.2 |
| 230 | 80 ± 5 | 1.04 ± 0.06 | 25.2 ± 0.2 | |
| 280 | 87 ± 6 | 0.96 ± 0.06 | 28.3 ± 0.2 | |
| BMNPs | 197 | 32 ± 3 | 0.50 ± 0.04 | 11.6 ± 0.2 |
| 230 | 36 ± 3 | 0.47 ± 0.04 | 12.9 ± 0.2 | |
| 280 | 47 ± 3 | 0.52 ± 0.03 | 16.7 ± 0.2 | |
| 25 B + 75 M | 197 | 73 ± 3 | 1.15 ± 0.05 | 28.5 ± 0.2 |
| 230 | 83 ± 3 | 1.08 ± 0.04 | 30.5 ± 0.2 | |
| 280 | 96 ± 2 | 1.26 ± 0.03 | 34.1 ± 0.2 | |
| 60 B + 40 M | 197 | 57 ± 4 | 0.90 ± 0.06 | 18.1 ± 0.2 |
| 230 | 62 ± 6 | 0.81 ± 0.07 | 21.6 ± 0.2 | |
| 280 | 75 ± 8 | 0.82 ± 0.09 | 27.5 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, G.R.; Jabalera, Y.; Peigneux, A.; Checa Fernández, B.L.; Delgado, Á.V.; Jimenez-Lopez, C. Enhancement of Magnetic Hyperthermia by Mixing Synthetic Inorganic and Biomimetic Magnetic Nanoparticles. Pharmaceutics 2019, 11, 273. https://doi.org/10.3390/pharmaceutics11060273
Iglesias GR, Jabalera Y, Peigneux A, Checa Fernández BL, Delgado ÁV, Jimenez-Lopez C. Enhancement of Magnetic Hyperthermia by Mixing Synthetic Inorganic and Biomimetic Magnetic Nanoparticles. Pharmaceutics. 2019; 11(6):273. https://doi.org/10.3390/pharmaceutics11060273
Chicago/Turabian StyleIglesias, Guillermo R., Ylenia Jabalera, Ana Peigneux, Blanca Luna Checa Fernández, Ángel V. Delgado, and Concepcion Jimenez-Lopez. 2019. "Enhancement of Magnetic Hyperthermia by Mixing Synthetic Inorganic and Biomimetic Magnetic Nanoparticles" Pharmaceutics 11, no. 6: 273. https://doi.org/10.3390/pharmaceutics11060273
APA StyleIglesias, G. R., Jabalera, Y., Peigneux, A., Checa Fernández, B. L., Delgado, Á. V., & Jimenez-Lopez, C. (2019). Enhancement of Magnetic Hyperthermia by Mixing Synthetic Inorganic and Biomimetic Magnetic Nanoparticles. Pharmaceutics, 11(6), 273. https://doi.org/10.3390/pharmaceutics11060273