Controlled Drug Delivery Systems for Oral Cancer Treatment—Current Status and Future Perspectives
Abstract
:1. Introduction
2. Anticancer Agents for Oral Cancer Treatment Formulated in Drug Delivery Systems
2.1. Paclitaxel (PTX)
2.2. Cisplatin (DDP)
2.3. Doxorubicin
2.4. Docetaxel
2.5. Methotrexate
2.6. Fluoropyrimidine 5-Fluorouracil
3. Carriers for OSCC Drug Delivery Systems
3.1. Nanoparticles for Drug Delivery
3.1.1. Polymeric Nanoparticles for Drug Delivery System
3.1.2. Inorganic Nanoparticles for Drug Delivery System
3.1.3. Combinational (Polymeric-Inorganic) Nanoparticles
3.2. Nanolipids
3.3. Hydrogel-Based Drug Delivery Systems
3.4. Exosomes
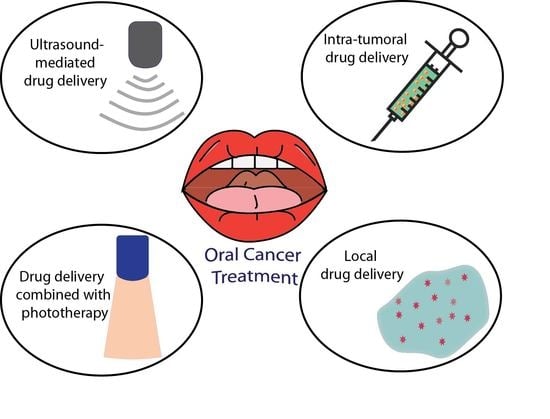
4. Controlled Drug Delivery Approaches for Oral Cancer
4.1. Intra-Tumoral Drug Delivery in Oral Cancer
4.2. Local Drug Delivery in Oral Cancer
4.3. Phototherapy Approaches in Drug Delivery
4.4. Microbubbles Mediated Ultrasound in Drug Delivery
5. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vogel, D.W.T.; Zbaeren, P.; Thoeny, H.C. Cancer of the oral cavity and oropharynx. Cancer Imaging 2010, 10, 62. [Google Scholar]
- Manikandan, M.; Rao, A.K.D.M.; Arunkumar, G.; Manickavasagam, M.; Rajkumar, K.S.; Rajaraman, R.; Munirajan, A.K. Oral squamous cell carcinoma: microRNA expression profiling and integrative analyses for elucidation of tumourigenesis mechanism. Mol. Cancer 2016, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884. [Google Scholar] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oral Cavity Cancer Statistics-Canadian Cancer Society. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/oral/statistics/?region=on (accessed on 28 June 2019).
- Denise, M.; Laronde, T.G.; Hislop, J.M.; Elwood, M.R. Oral Cancer: Just the Facts-Canadian Dental Association. Available online: https://cda-adc.ca/jcda/vol-74/issue-3/269.pdf (accessed on 28 June 2019).
- Marur, S.; Forastiere, A.A. Head and neck cancer: Changing epidemiology, diagnosis, and treatment. Mayo Clin. Proc. 2008, 83, 489–501. [Google Scholar] [CrossRef]
- Nör, J.E.; Gutkind, J.S. Head and neck cancer in the new era of precision medicine. J. Dent. Res. 2018, 97, 601–602. [Google Scholar] [CrossRef] [PubMed]
- Prince, V.M.; Papagerakis, S.; Prince, M.E. Oral Cancer and Cancer Stem Cells: Relevance to Oral Cancer Risk Factors, Premalignant Lesions, and Treatment. Curr. Oral Heal. Rep. 2016, 3, 65–73. [Google Scholar] [CrossRef]
- Heck, J.E.; Berthiller, J.; Vaccarella, S.; Winn, D.M.; Smith, E.M.; Shan’gina, O.; Schwartz, S.M.; Purdue, M.P.; Pilarska, A.; Eluf-Neto, J. Sexual behaviours and the risk of head and neck cancers: a pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int. J. Epidemiol. 2009, 39, 166–181. [Google Scholar] [CrossRef] [Green Version]
- Majchrzak, E.; Szybiak, B.; Wegner, A.; Pienkowski, P.; Pazdrowski, J.; Luczewski, L.; Sowka, M.; Golusinski, P.; Malicki, J.; Golusinski, W. Oral cavity and oropharyngeal squamous cell carcinoma in young adults: a review of the literature. Radiol. Oncol. 2014, 48, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pickard, R.K.L.; Xiao, W.; Broutian, T.R.; He, X.; Gillison, M.L. The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18–30 years. Sex. Transm. Dis. 2012, 39, 559–566. [Google Scholar] [CrossRef]
- Nirvani, M.; Khuu, C.; Utheim, T.P.; Sand, L.P.; Sehic, A. Circadian clock and oral cancer. Mol. Clin. Oncol. 2018, 8, 219–226. [Google Scholar] [PubMed]
- Hsu, C.; Lin, S.; Lu, C.; Lin, P.; Yang, M. Altered expression of circadian clock genes in head and neck squamous cell carcinoma. Tumor Biol. 2012, 33, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Adeola, H.A.; Papagerakis, P.; Papagerakis, S. System Biology approaches and Precision Oral Health: a Circadian Clock Perspective. Front. Physiol. 2019, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/home (accessed on 28 June 2019).
- Adams, A.K.; Hallenbeck, G.E.; Casper, K.A.; Patil, Y.J.; Wilson, K.M.; Kimple, R.J.; Lambert, P.F.; Witte, D.P.; Xiao, W.; Gillison, M.L. DEK promotes HPV-positive and-negative head and neck cancer cell proliferation. Oncogene 2015, 34, 868. [Google Scholar] [CrossRef] [PubMed]
- Hübbers, C.U.; Akgül, B. HPV and cancer of the oral cavity. Virulence 2015, 6, 244–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouda, M.; Gorgoulis, V.G.; Kastrinakis, N.G.; Giannoudis, A.; Tsoli, E.; Danassi-Afentaki, D.; Foukas, P.; Kyroudi, A.; Laskaris, G.; Herrington, C.S. “High risk” HPV types are frequently detected in potentially malignant and malignant oral lesions, but not in normal oral mucosa. Mod. Pathol. 2000, 13, 644. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Maeda, H.; Sugita, Y.; Tanaka, S.; Kameyama, Y. Human papillomavirus type 38 infection in oral squamous cell carcinomas. Oral Oncol. 2002, 38, 591–596. [Google Scholar] [CrossRef]
- Feller, L.; Wood, N.H.; Khammissa, R.A.G.; Lemmer, J. Human papillomavirus-mediated carcinogenesis and HPV-associated oral and oropharyngeal squamous cell carcinoma. Part 2: Human papillomavirus associated oral and oropharyngeal squamous cell carcinoma. Head Face Med. 2010, 6, 15. [Google Scholar] [CrossRef]
- Feller, L.; Wood, N.H.; Khammissa, R.A.G.; Lemmer, J. Human papillomavirus-mediated carcinogenesis and HPV-associated oral and oropharyngeal squamous cell carcinoma. Part 1: Human papillomavirus-mediated carcinogenesis. Head Face Med. 2010, 6, 14. [Google Scholar] [CrossRef]
- Pinatti, L.M.; Walline, H.M.; Carey, T.E. Human papillomavirus genome integration and head and neck cancer. J. Dent. Res. 2018, 97, 691–700. [Google Scholar] [CrossRef]
- Gillison, M.L.; Broutian, T.; Pickard, R.K.L.; Tong, Z.; Xiao, W.; Kahle, L.; Graubard, B.I.; Chaturvedi, A.K. Prevalence of oral HPV infection in the United States, 2009–2010. Jama 2012, 307, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Villa, A.; Nyitray, A.G.; Abrahamsen, M.; Papenfuss, M.; Smith, D.; Hildesheim, A.; Villa, L.L.; Lazcano-Ponce, E.; Giuliano, A.R. The epidemiology of oral HPV infection among a multinational sample of healthy men. Cancer Epidemiol. Prev. Biomark. 2011, 20, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Rabinovics, N.; Mizrachi, A.; Hadar, T.; Ad-El, D.; Feinmesser, R.; Guttman, D.; Shpitzer, T.; Bachar, G. Cancer of the head and neck region in solid organ transplant recipients. Head Neck 2014, 36, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.W.; Day, T.A. Oral cancer and precancerous lesions. CA. Cancer J. Clin. 2002, 52, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Furness, S.; Glenny, A.-M.; Worthington, H.V.; Pavitt, S.; Oliver, R.; Clarkson, J.E.; Macluskey, M.; Chan, K.K.; Conway, D.I. The CSROC Expert Panel Interventions for the treatment of oral cavity and oropharyngeal cancer: chemotherapy. In The Cochrane Database of Systematic Reviews; Furness, S., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2010; p. CD006386. [Google Scholar]
- Moskovitz, J.; Moy, J.; Ferris, R.L. Immunotherapy for head and neck squamous cell carcinoma. Curr. Oncol. Rep. 2018, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Rapidis, A.D.; Wolf, G.T. Immunotherapy of head and neck cancer: Current and future considerations. J. Oncol. 2009, 2009, 346345. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y. Overview of Current Cancer Immunotherapy. In Immunotherapy of Cancer: An Innovative Treatment Comes of Age; Yamaguchi, Y., Ed.; Springer Japan: Tokyo, Japan, 2016; pp. 3–17. ISBN 978-4-431-55031-0. [Google Scholar]
- Cheng, C.-T.; Castro, G.; Liu, C.-H.; Lau, P. Advanced nanotechnology: An arsenal to enhance immunotherapy in fighting cancer. Clin. Chim. Acta 2019, 492, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Musetti, S.N.; Huang, L. Nanomaterials for cancer immunotherapy. Biomaterials 2017, 148, 16–30. [Google Scholar] [CrossRef]
- Khalil, D.N.; Budhu, S.; Gasmi, B.; Zappasodi, R.; Hirschhorn-Cymerman, D.; Plitt, T.; De Henau, O.; Zamarin, D.; Holmgaard, R.B.; Murphy, J.T. The new era of cancer immunotherapy: manipulating T-cell activity to overcome malignancy. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2015; Volume 128, pp. 1–68. ISBN 0065-230X. [Google Scholar]
- Lubek, J.E. Head and Neck Cancer Research and Support Foundations. Oral Maxillofac. Surg. Clin. 2018, 30, 459–469. [Google Scholar] [CrossRef]
- Colevas, A.D.; Yom, S.S.; Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J. NCCN guidelines insights: Head and neck cancers, version 1.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Sim, F.; Leidner, R.; Bell, R.B. Immunotherapy for Head and Neck Cancer. Oral Maxillofac. Surg. Clin. 2019, 31, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.C.; Bakkenist, C.J.; Ferris, R.L.; Clump, D.A. Role of immunotherapy in head and neck cancer. Semin. Radiat. Oncol. 2018, 28, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.M.; Ferris, R.L. Tumor Immunology, Immunotherapy and Its Application to Head and Neck Squamous Cell Carcinoma (HNSCC). In Critical Issues in Head and Neck Oncology; Springer International Publishing: Cham, Switzerland, 2018; pp. 341–355. [Google Scholar]
- Chowdhury, M.M.H.; Kubra, K.; Kanwar, R.K.; Kanwar, J.R. Nanoparticles Advancing Cancer Immunotherapy. In Biomedical Applications of Graphene and 2D Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–304. [Google Scholar]
- Hirabayashi, F.; Iwanaga, K.; Okinaga, T.; Takahashi, O.; Ariyoshi, W.; Suzuki, R.; Sugii, M.; Maruyama, K.; Tominaga, K.; Nishihara, T. Epidermal growth factor receptor-targeted sonoporation with microbubbles enhances therapeutic efficacy in a squamous cell carcinoma model. PLoS ONE 2017, 12, e0185293. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Tominaga, K.; Iwanaga, K.; Nagao, F.; Habu, M.; Tsujisawa, T.; Seta, Y.; Toyoshima, K.; Fukuda, J.; Nishihara, T. Targeted drug delivery system for oral cancer therapy using sonoporation. J. Oral Pathol. Med. 2009, 38, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Karavasili, C.; Andreadis, D.A.; Katsamenis, O.L.; Panteris, E.; Anastasiadou, P.; Kakazanis, Z.; Zoumpourlis, V.; Markopoulou, C.K.; Koutsopoulos, S.; Vizirianakis, I.S.; et al. Synergistic Antitumor Potency of a Self-Assembling Peptide Hydrogel for the Local Co-delivery of Doxorubicin and Curcumin in the Treatment of Head and Neck Cancer. Mol. Pharm. 2019, 16, 2326–2341. [Google Scholar] [CrossRef] [PubMed]
- Papagerakis, S.; Bellile, E.; Peterson, L.A.; Pliakas, M.; Balaskas, K.; Selman, S.; Hanauer, D.; Taylor, J.M.G.; Duffy, S.; Wolf, G. Proton pump inhibitors and histamine 2 blockers are associated with improved overall survival in patients with head and neck squamous carcinoma. Cancer Prev. Res. 2014, 7, 1258–1269. [Google Scholar] [CrossRef]
- Desiderio, V.; Papagerakis, P.; Tirino, V.; Zheng, L.; Matossian, M.; Prince, M.E.; Paino, F.; Mele, L.; Papaccio, F.; Montella, R. Increased fucosylation has a pivotal role in invasive and metastatic properties of head and neck cancer stem cells. Oncotarget 2015, 6, 71. [Google Scholar] [CrossRef]
- Matossian, M.; Vangelderen, C.; Papagerakis, P.; Zheng, L.; Wolf, G.T.; Papagerakis, S. In silico modeling of the molecular interactions of antacid medication with the endothelium: novel therapeutic implications in head and neck carcinomas. Int. J. Immunopathol. Pharmacol. 2014, 27, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F.; Okyar, A. Circadian clocks and drug delivery systems: Impact and opportunities in chronotherapeutics. Expert Opin. Drug Deliv. 2011, 8, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F.; Schibler, U. Circadian rhythms: Mechanisms and therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 593–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchiya, Y.; Ushijima, K.; Noguchi, T.; Okada, N.; Hayasaka, J.; Jinbu, Y.; Ando, H.; Mori, Y.; Kusama, M.; Fujimura, A. Influence of a dosing-time on toxicities induced by docetaxel, cisplatin and 5-fluorouracil in patients with oral squamous cell carcinoma; a cross-over pilot study. Chronobiol. Int. 2018, 35, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K.; Misra, R.; Sahoo, S.K. Nanoparticles: A Boon to Drug Delivery, Therapeutics, Diagnostics and Imaging. In Nanomedicine in Cancer; Pan Stanford: Singapore, 2017; pp. 47–98. [Google Scholar]
- FDA Approved Drugs in Oncology|CenterWatch. Available online: https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology (accessed on 28 June 2019).
- O’neill, V.J.; Twelves, C.J. Oral cancer treatment: developments in chemotherapy and beyond. Br. J. Cancer 2002, 87, 933. [Google Scholar] [CrossRef]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef]
- Pridgen, E.M.; Alexis, F.; Farokhzad, O.C. Polymeric nanoparticle drug delivery technologies for oral delivery applications. Expert Opin. Drug Deliv. 2015, 12, 1459–1473. [Google Scholar] [CrossRef] [Green Version]
- Chemotherapy for Oral Cavity and Oropharyngeal Cancer. Available online: https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/treating/chemotherapy.html (accessed on 28 June 2019).
- Choi, J.-S. Pharmacokinetics of paclitaxel in rabbits with carbon tetrachloride-lnduced hepatic failure. Arch. Pharm. Res. 2002, 25, 973–977. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.; Lee, I.-H.; Yu, M.; Kim, H.; Chae, S.Y.; Jon, S. Conjugated chitosan as a novel platform for oral delivery of paclitaxel. J. Med. Chem. 2008, 51, 6442–6449. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Amiji, M.M. Improved oral delivery of paclitaxel following administration in nanoemulsion formulations. J. Nanosci. Nanotechnol. 2006, 6, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Feng, S.-S. Poly(d,l-lactide-co-glycolide)/montmorillonite nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 6068–6076. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Ike, O.; Wada, H.; Hitomi, S.; Amano, Y.; Ogita, I.; Nakai, N.; Takada, K. Oral sustained-release cisplatin preparation for rats and mice. J. Pharm. Pharmacol. 1997, 49, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.; Peng, S.; Xu, C.; Sun, S. Porous hollow Fe3O4 nanoparticles for targeted delivery and controlled release of cisplatin. J. Am. Chem. Soc. 2009, 131, 10637–10644. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gemeinhart, R.A. Cisplatin delivery from poly(acrylic acid-co-methyl methacrylate) microparticles. J. Control. Release 2005, 106, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Nanjwade, B.K.; Singh, J.; Parikh, K.A.; Manvi, F.V. Preparation and evaluation of carboplatin biodegradable polymeric nanoparticles. Int. J. Pharm. 2010, 385, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Astra, L.I.; Hammond, R.; Tarakji, K.; Stephenson, L.W. Doxorubicin-Induced Canine CHF: Advantages and Disadvantages 1. J. Card. Surg. 2003, 18, 301–306. [Google Scholar] [CrossRef]
- Christiansen, S.; Autschbach, R. Doxorubicin in experimental and clinical heart failure. Eur. J. Cardio-Thoracic Surg. 2006, 30, 611–616. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhu, L.; Liu, Z.; Cheng, R.; Meng, F.; Cui, J.; Ji, S.; Zhong, Z. Reversibly stabilized multifunctional dextran nanoparticles efficiently deliver doxorubicin into the nuclei of cancer cells. Angew. Chem. 2009, 121, 10098–10102. [Google Scholar] [CrossRef]
- She, W.; Li, N.; Luo, K.; Guo, C.; Wang, G.; Geng, Y.; Gu, Z. Dendronized heparin− doxorubicin conjugate based nanoparticle as pH-responsive drug delivery system for cancer therapy. Biomaterials 2013, 34, 2252–2264. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.F.; Hussain, S.Z.; Saeed, H.; Javed, I.; Sarwar, H.S.; Nadhman, A.; Rehman, M.; Jahan, S.; Hussain, I.; Shahnaz, G. Polymeric nanocapsules embedded with ultra-small silver nanoclusters for synergistic pharmacology and improved oral delivery of Docetaxel. Sci. Rep. 2018, 8, 13304. [Google Scholar] [CrossRef] [PubMed]
- Jolivet, J.; Cowan, K.H.; Curt, G.A.; Clendeninn, N.J.; Chabner, B.A. The pharmacology and clinical use of methotrexate. New Engl. J. Med. 1983, 309, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- McLean-Tooke, A.; Aldridge, C.; Waugh, S.; Spickett, G.P.; Kay, L. Methotrexate, rheumatoid arthritis and infection risk—What is the evidence? Rheumatology 2009, 48, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.A.; Perrier, D.G.; Dorr, R.T.; Alberts, D.S.; Finley, P.R. Methotrexate: bioavailability and pharmacokinetics. Cancer Treat. Rep. 1985, 69, 833–838. [Google Scholar] [PubMed]
- Kumar, A.B.M.; Rao, K.P. Preparation and characterization of pH-sensitive proteinoid microspheres for the oral delivery of methotrexate. Biomaterials 1998, 19, 725–732. [Google Scholar] [CrossRef]
- Paliwal, R.; Rai, S.; Vaidya, B.; Khatri, K.; Goyal, A.K.; Mishra, N.; Mehta, A.; Vyas, S.P. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 184–191. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Chen, G.; Wei, P.; Ping, Q. PLGA nanoparticles for the oral delivery of 5-Fluorouracil using high pressure homogenization-emulsification as the preparation method and in vitro/in vivo studies. Drug Dev. Ind. Pharm. 2008, 34, 107–115. [Google Scholar] [CrossRef]
- Jawahar, N.; Meyyanathan, S. Polymeric nanoparticles for drug delivery and targeting: A comprehensive review. Int. J. Heal. Allied Sci. 2012, 1, 217. [Google Scholar] [CrossRef]
- Sim, R.B.; Wallis, R. Surface properties: Immune attack on nanoparticles. Nat. Nanotechnol. 2011, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Feng, S.S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Huang, H.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic nanoparticles for cancer imaging and therapy. J. Control. Release 2011, 155, 344–357. [Google Scholar] [CrossRef]
- Subramani, K.; Ahmed, W. Nanoparticulate Drug Delivery Systems for Oral Cancer Treatment, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; ISBN 9781455778621. [Google Scholar]
- Sato, I.; Umemura, M.; Mitsudo, K.; Fukumura, H.; Kim, J.H.; Hoshino, Y.; Nakashima, H.; Kioi, M.; Nakakaji, R.; Sato, M.; et al. Simultaneous hyperthermia-chemotherapy with controlled drug delivery using single-drug nanoparticles. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar]
- Khosa, A.; Reddi, S.; Saha, R.N. Biomedicine & Pharmacotherapy Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar]
- Calixto, G.; Bernegossi, J.; Fonseca-Santos, B.; Chorilli, M. Nanotechnology-based drug delivery systems for treatment of oral cancer: A review. Int. J. Nanomed. 2014, 9, 3719–3735. [Google Scholar] [CrossRef]
- Sun, M.; Su, X.; Ding, B.; He, X.; Liu, X.; Yu, A.; Lou, H.; Zhai, G. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine 2012, 7, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Lohani, A.; Bhattacharya, S.S. Hydrogel as a novel drug delivery system: A review. J. Fundam. Pharm. Res. 2014, 2, 35–48. [Google Scholar]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poonia, M.; Ramalingam, K.; Goyal, S.; Sidhu, K.S. Nanotechnology in oral cancer: A comprehensive review. J. Oral Maxillofac. Pathol. 2017, 3, 407–414. [Google Scholar]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 206–212. [Google Scholar] [CrossRef]
- Brewer, E.; Coleman, J.; Lowman, A. Emerging technologies of polymeric nanoparticles in cancer drug delivery. J. Nanomater. 2011, 2011, 10. [Google Scholar] [CrossRef]
- Desai, K.G.H. Polymeric drug delivery systems for intraoral site-specific chemoprevention of oral cancer. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1383–1413. [Google Scholar] [CrossRef]
- Endo, K.; Ueno, T.; Kondo, S.; Wakisaka, N.; Murono, S.; Ito, M.; Kataoka, K.; Kato, Y.; Yoshizaki, T. Tumor-targeted chemotherapy with the nanopolymer-based drug NC-6004 for oral squamous cell carcinoma. Cancer Sci. 2013, 104, 369–374. [Google Scholar] [CrossRef]
- Madhulaxmi, M.; Iyer, K.; Periasamy, R.; Gajendran, P.; Lakshmi, T. Role of cisplatin in oral squamous cell carcinoma—A review. J. Adv. Pharm. Educ. Res. 2017, 7, 39–42. [Google Scholar]
- Uchino, H.; Matsumura, Y.; Negishi, T.; Koizumi, F.; Hayashi, T.; Honda, T.; Nishiyama, N.; Kataoka, K.; Naito, S.; Kakizoe, T. Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br. J. Cancer 2005, 93, 678. [Google Scholar] [CrossRef] [PubMed]
- Mazzarino, L.; Loch-neckel, G.; Bubniak, S.; Mazzucco, S.; Santos-silva, M.C.; Borsali, R.; Lemos-senna, E. Curcumin-loaded chitosan-coated nanoparticles as a new approach for the local treatment of oral cavity cancer. J. Nanosci. Nanotechnol. 2015, 15, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Mazzarino, L.; Travelet, C.; Ortega-Murillo, S.; Otsuka, I.; Pignot-Paintrand, I.; Lemos-Senna, E.; Borsali, R. Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J. Colloid Interface Sci. 2012, 370, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.G.; Mazzarino, L.; Dora, C.L.; Bellettini, I.C.; Minatti, E.; Lemos-Senna, E. del documento: Curcumin-loaded polymeric and lipid nanocapsules: Preparation, characterization and chemical stability evaluation. Indizada en Chem. Abstr. Serv. Int. Pharm. Abstr. Serv. Biosci. Inf. Serv. (Biol. Abstr. Period. Int. Pharm. Technol. Prod. Manuf. Abstr. Ref. Zhurnal EMBAS) 2002, 29, 933–940. [Google Scholar]
- Weisburg, J.H.; Schuck, A.G.; Reiss, S.E.; Wolf, B.J.; Fertel, S.R.; Zuckerbraun, H.L.; Babich, H. Ellagic acid, a dietary polyphenol, selectively cytotoxic to HSC-2 oral carcinoma cells. Anticancer Res. 2013, 33, 1829–1836. [Google Scholar] [PubMed]
- Arulmozhi, V.; Pandian, K.; Mirunalini, S. Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids Surf. B Biointerfaces 2013, 110, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef]
- Lucky, S.S.; Idris, N.M.; Huang, K.; Kim, J.; Li, Z.; Thong, P.S.P.; Xu, R.; Soo, K.C.; Zhang, Y. In vivo biocompatibility, biodistribution and therapeutic efficiency of titania coated upconversion nanoparticles for photodynamic therapy of solid oral cancers. Theranostics 2016, 6, 1844–1865. [Google Scholar] [CrossRef]
- Marcazzan, S.; Varoni, E.M.; Blanco, E.; Lodi, G.; Ferrari, M. Nanomedicine, an emerging therapeutic strategy for oral cancer therapy. Oral Oncol. 2018, 76, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, H.; Umemura, M.; Kurotani, R.; Fukumura, H.; Sato, I.; Kim, J.-H.; Hoshino, Y.; Lee, J.; Amemiya, N.; Sato, M. A magnetic anti-cancer compound for magnet-guided delivery and magnetic resonance imaging. Sci. Rep. 2015, 5, 9194. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, X.; Zhang, K.; Sun, B.; Wang, L.; Meng, L.; Liu, Q.; Zheng, C.; Yang, B.; Sun, H. Codelivery of doxorubicin and MDR1-siRNA by mesoporous silica nanoparticles-polymerpolyethylenimine to improve oral squamous carcinoma treatment. Int. J. Nanomed. 2018, 13, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.L.; Li, Y.; Zhao, L.M.; Su, L.W.; Ding, G. Delivery of MTH1 inhibitor (TH287) and MDR1 siRNA via hyaluronic acid-based mesoporous silica nanoparticles for oral cancers treatment. Colloids Surf. B Biointerfaces 2019, 173, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.R.; Siddharth, S.; Das, D.; Nayak, A.; Kundu, C.N. Enhancement of Cytotoxicity and Inhibition of Angiogenesis in Oral Cancer Stem Cells by a Hybrid Nanoparticle of Bioactive Quinacrine and Silver: Implication of Base Excision Repair Cascade. Mol. Pharm. 2015, 12, 4011–4025. [Google Scholar] [CrossRef] [PubMed]
- Rana, V. Therapeutic Delivery. Ther. Deliv 2016, 7, 117–138. [Google Scholar]
- Sah, A.K.; Vyas, A.; Suresh, P.K.; Gidwani, B. Application of nanocarrier-based drug delivery system in treatment of oral cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.S.; Dathar, S. Nano drug delivery in oral cancer therapy: An emerging avenue to unveil. J. Med. Radiol. Pathol. Surg. 2015, 1, 17–22. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-L.; Al-Suwayeh, S.A.; Fang, J.-Y. Nanostructured Lipid Carriers (NLCs) for Drug Delivery and Targeting. Recent Pat. Nanotechnol. 2012, 7, 41–55. [Google Scholar] [CrossRef]
- Zlotogorski, A.; Dayan, A.; Dayan, D.; Chaushu, G.; Salo, T.; Vered, M. Nutraceuticals as new treatment approaches for oral cancer-I: Curcumin. Oral Oncol. 2013, 49, 187–191. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Z.; Wang, L.; Zhang, C.; Zhang, N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf. B Biointerfaces 2011, 85, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Iida, S.; Shimada, J.; Sakagami, H. Cytotoxicity induced by docetaxel in human oral squamous cell carcinoma cell lines. In Vivo (Brooklyn) 2013, 27, 321–332. [Google Scholar]
- Zhang, T.; Chen, J.; Zhang, Y.; Shen, Q.; Pan, W. Characterization and evaluation of nanostructured lipid carrier as a vehicle for oral delivery of etoposide. Eur. J. Pharm. Sci. 2011, 43, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Kumar Shukla, V. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 2014, 25–31. [Google Scholar]
- Ketabat, F.; Khorshidi, S.; Karkhaneh, A. Application of minimally invasive injectable conductive hydrogels as stimulating scaffolds for myocardial tissue engineering. Polym. Int. 2018, 67, 975–982. [Google Scholar] [CrossRef]
- Ketabat, F.; Karkhaneh, A.; Mehdinavaz Aghdam, R.; Hossein Ahmadi Tafti, S. Injectable conductive collagen/alginate/polypyrrole hydrogels as a biocompatible system for biomedical applications. J. Biomater. Sci. Polym. Ed. 2017, 28, 794–805. [Google Scholar] [CrossRef]
- Sepantafar, M.; Maheronnaghsh, R.; Mohammadi, H.; Radmanesh, F.; Hasani-sadrabadi, M.M.; Ebrahimi, M.; Baharvand, H. Engineered Hydrogels in Cancer Therapy and Diagnosis. Trends Biotechnol. 2017, 35, 1074–1087. [Google Scholar] [CrossRef]
- Multifunctional Nanoparticles for Drug Delivery Applications; Svenson, S.; Prud’homme, R.K. (Eds.) Nanostructure Science and Technology; Springer US: Boston, MA, USA, 2012; ISBN 978-1-4614-2304-1. [Google Scholar]
- Svenson, S.; Prud’homme, R.K. Multifunctional Nanoparticles for Drug Delivery Applications: Imaging, Targeting, and Delivery; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 146142304X. [Google Scholar]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Karavasili, C.; Panteris, E.; Vizirianakis, I.S.; Koutsopoulos, S.; Fatouros, D.G. Chemotherapeutic Delivery from a Self-Assembling Peptide Nanofiber Hydrogel for the Management of Glioblastoma. Pharm. Res. 2018, 35, 166. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Unsworth, L.D.; Nagai, Y.; Zhang, S. Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proc. Natl. Acad. Sci. 2009, 106, 4623–4628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutsopoulos, S.; Zhang, S. Two-layered injectable self-assembling peptide scaffold hydrogels for long-term sustained release of human antibodies. J. Control. Release 2012, 160, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, C.; Feng, X.; Zhou, X.; Xu, X.; Xie, L.; Wang, R.; Zhang, D.; Wang, H.; Deng, P. Biodegradable thermosensitive hydrogel for SAHA and DDP delivery: therapeutic effects on oral squamous cell carcinoma xenografts. PLoS ONE 2012, 7, e33860. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteomics 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.-C.; Gao, J.-Q. Exosomes as novel bio-carriers for gene and drug delivery. Int. J. Pharm. 2017, 521, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, G.; Bunggulawa, E.J.; Wang, N.; Yin, T.; Wang, Y.; Durkan, C. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnol. 2018, 16, 1–13. [Google Scholar] [Green Version]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehari, H.; Ito, Y.; Nakamura, T.; Kobune, M.; Sasaki, K.; Yonekura, N.; Kohama, G.; Hamada, H. Enhanced antitumor effect of RGD fiber-modified adenovirus for gene therapy of oral cancer. Cancer Gene Ther. 2003, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Wenig, B.L.; Werner, J.A.; Castro, D.J.; Sridhar, K.S.; Garewal, H.S.; Kehrl, W.; Pluzanska, A.; Arndt, O.; Costantino, P.D.; Mills, G.M. The role of intratumoral therapy with cisplatin/epinephrine injectable gel in the management of advanced squamous cell carcinoma of the head and neck. Arch. Otolaryngol. Neck Surg. 2002, 128, 880–885. [Google Scholar] [CrossRef]
- Minko, T.; Dharap, S.S.; Pakunlu, R.I.; Wang, Y. Molecular targeting of drug delivery systems to cancer. Curr. Drug Targets 2004, 5, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-T.; Zhou, S.-H. Nanoparticle-based targeted therapeutics in head-and-neck cancer. Int. J. Med. Sci. 2015, 12, 187. [Google Scholar] [CrossRef]
- Sankar, V.; Hearnden, V.; Hull, K.; Juras, D.V.; Greenberg, M.S.; Kerr, A.R.; Lockhart, P.B.; Patton, L.L.; Porter, S.; Thornhill, M. Local drug delivery for oral mucosal diseases: Challenges and opportunities. Oral Dis. 2011, 17, 73–84. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D. Photodynamic therapy of cancer: An update. CA. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Guo, R.; Peng, H.; Tian, Y.; Shen, S.; Yang, W. Mitochondria-targeting magnetic composite nanoparticles for enhanced phototherapy of cancer. Small 2016, 12, 4541–4552. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, Y.; Zhang, W.; Hao, Y.; Wang, Y.; Zhang, H.; Hou, L.; Zhang, Z. Programmed near-infrared light-responsive drug delivery system for combined magnetic tumor-targeting magnetic resonance imaging and chemo-phototherapy. Acta Biomater. 2017, 49, 402–413. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, Y.; Zhang, W.; Shan, X.; Yuan, Y.; Zhang, H.; Hou, L.; Zhang, Z. Tumor-targeted and multi-stimuli responsive drug delivery system for near-infrared light induced chemo-phototherapy and photoacoustic tomography. Acta Biomater. 2016, 38, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Detrembleur, C.; De Pauw-Gillet, M.; Mornet, S.; Jérôme, C.; Duguet, E. Gold Nanorods Coated with Mesoporous Silica Shell as Drug Delivery System for Remote Near Infrared Light-Activated Release and Potential Phototherapy. Small 2015, 11, 2323–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einafshar, E.; Asl, A.H.; Nia, A.H.; Mohammadi, M.; Malekzadeh, A.; Ramezani, M. New cyclodextrin-based nanocarriers for drug delivery and phototherapy using an irinotecan metabolite. Carbohydr. Polym. 2018, 194, 103–110. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, D.; Lin, W. Self-assembled core–shell nanoparticles for combined chemotherapy and photodynamic therapy of resistant head and neck cancers. ACS Nano 2015, 9, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Sennoga, C.A.; Kanbar, E.; Auboire, L.; Dujardin, P.-A.; Fouan, D.; Escoffre, J.-M.; Bouakaz, A. Microbubble-mediated ultrasound drug-delivery and therapeutic monitoring. Expert Opin. Drug Deliv. 2017, 14, 1031–1043. [Google Scholar] [CrossRef]
- Ibsen, S.; Schutt, C.E.; Esener, S. Microbubble-mediated ultrasound therapy: a review of its potential in cancer treatment. Drug Des. Devel. Ther. 2013, 7, 375. [Google Scholar] [CrossRef] [PubMed]
- Sorace, A.G.; Warram, J.M.; Umphrey, H.; Hoyt, K. Microbubble-mediated ultrasonic techniques for improved chemotherapeutic delivery in cancer. J. Drug Target. 2012, 20, 43–54. [Google Scholar] [CrossRef]
- Carson, A.R.; McTiernan, C.F.; Lavery, L.; Grata, M.; Leng, X.; Wang, J.; Chen, X.; Villanueva, F.S. Ultrasound-targeted microbubble destruction to deliver siRNA cancer therapy. Cancer Res. 2012, 72, 6191–6199. [Google Scholar] [CrossRef]
- Kerr, W.G.; Chisholm, J.D. The Next Generation of Immunotherapy for Cancer: Small Molecules Could Make Big Waves. J. Immunol. 2019, 202, 11–19. [Google Scholar] [CrossRef]
- Zhu, H.-F.; Li, Y. Small-molecule targets in tumor immunotherapy. Nat. Prod. Bioprospect. 2018, 8, 297–301. [Google Scholar] [CrossRef]
- Van Nimwegen, S.A.; Bakker, R.C.; Kirpensteijn, J.; van Es, R.J.J.; Koole, R.; Lam, M.; Hesselink, J.W.; Nijsen, J.F.W. Intratumoral injection of radioactive holmium (166Ho) microspheres for treatment of oral squamous cell carcinoma in cats. Vet. Comp. Oncol. 2018, 16, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Singh, M.; Kumar, R.; Belz, J.; Shanker, R.; Dwivedi, P.D.; Sridhar, S.; Singh, S.P. Synthesis and in vitro studies of PLGA-DTX nanoconjugate as potential drug delivery vehicle for oral cancer. Int. J. Nanomed. 2018, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, J.-H.; Liu, Q.; Huang, H.; Chen, M.; Li, K.; Li, C.; Yu, X.-F.; Chu, P.K. Rose-bengal-conjugated gold nanorods for in vivo photodynamic and photothermal oral cancer therapies. Biomaterials 2014, 35, 1954–1966. [Google Scholar] [CrossRef] [PubMed]
- Gavin, A.; Pham, J.T.H.; Wang, D.; Brownlow, B.; Elbayoumi, T.A. Layered nanoemulsions as mucoadhesive buccal systems for controlled delivery of oral cancer therapeutics. Int. J. Nanomedicine 2015, 10, 1569. [Google Scholar] [PubMed]
- Jin, B.; Dong, X.; Xu, X.; Zhang, F. Development and in vitro evaluation of mucoadhesive patches of methotrexate for targeted delivery in oral cancer. Oncol. Lett. 2018, 15, 2541–2549. [Google Scholar] [CrossRef]
- Wang, D.; Fei, B.; Halig, L.V.; Qin, X.; Hu, Z.; Xu, H.; Wang, Y.A.; Chen, Z.; Kim, S.; Shin, D.M. Targeted iron-oxide nanoparticle for photodynamic therapy and imaging of head and neck cancer. ACS Nano 2014, 8, 6620–6632. [Google Scholar] [CrossRef]
- Kozakiewicz, P.; Grzybowska-Szatkowska, L. Application of molecular targeted therapies in the treatment of head and neck squamous cell carcinoma. Oncol. Lett. 2018, 15, 7497–7505. [Google Scholar] [CrossRef]
- Razak, A.R.A.; Ahn, M.-J.; Yen, C.-J.; Solomon, B.J.; Lee, S.-H.; Wang, H.-M.; Munster, P.N.; Van Herpen, C.M.L.; Gilbert, J.; Pal, R.R.; et al. Phase lb/ll study of the PI3Kα inhibitor BYL719 in combination with cetuximab in recurrent/metastatic squamous cell cancer of the head and neck (SCCHN). J. Clin. Oncol. 2014, 32, 6044. [Google Scholar] [CrossRef]
- Dietsch, G.N.; Lu, H.; Yang, Y.; Morishima, C.; Chow, L.Q.; Disis, M.L.; Hershberg, R.M. Coordinated Activation of Toll-Like Receptor8 (TLR8) and NLRP3 by the TLR8 Agonist, VTX-2337, Ignites Tumoricidal Natural Killer Cell Activity. PLoS ONE 2016, 11, e0148764. [Google Scholar] [CrossRef]
- WEE1 Inhibitor With Cisplatin and Radiotherapy: A Trial in Head and Neck Cancer. Available online: https://clinicaltrials.gov (accessed on 23 May 2019).
- Clinical Trial of Abemaciclib in Combination with Pembrolizumab in Patients with Metastatic or Recurrent Head and Neck Cancer. Available online: https://clinicaltrials.gov (accessed on 23 May 2019).
- TPST-1120 as Monotherapy and in Combination with (Nivolumab, Docetaxel or Cetuximab) in Subjects with Advanced Cancers. Available online: https://clinicaltrials.gov (accessed on 23 May 2019).
- Sitravatinib (MGCD516) and Nivolumab in Oral Cavity Cancer Window Opportunity Study. Available online: https://clinicaltrials.gov (accessed on 23 May 2019).
- Trial of BIBF1120 (Nintedanib) in Patients with Recurrent or Metastatic Salivary Gland Cancer of the Head and Neck. Available online: https://clinicaltrials.gov (accessed on 23 May 2019).
- Azacitidine, Durvalumab, and Tremelimumab in Recurrent and/or Metastatic Head and Neck Cancer Patients. Available online: https://clinicaltrials.gov (accessed on 23 May 2019).
- Safety and Efficacy of MEDI0457 and Durvalumab in Patients with HPV Associated Recurrent/Metastatic Head and Neck Cancer. Available online: https://clinicaltrials.gov (accessed on 23 May 2019).
- Phase III Open Label Study of MEDI 4736 With/Without Tremelimumab Versus Standard of Care (SOC) in Recurrent/Metastatic Head and Neck Cancer. Available online: https://clinicaltrials.gov (accessed on 23 May 2019).
- Shah, J.P.; Gil, Z. Current concepts in management of oral cancer–surgery. Oral Oncol. 2009, 45, 394–401. [Google Scholar] [CrossRef]
- Okunaga, S.; Takasu, A.; Meshii, N.; Imai, T.; Hamada, M.; Iwai, S.; Yura, Y. Ultrasound as a method to enhance antitumor ability of oncolytic herpes simplex virus for head and neck cancer. Cancer Gene Ther. 2015, 22, 163. [Google Scholar] [CrossRef] [PubMed]
- Drugs@FDA: FDA Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed on 23 May 2019).
- Muggia, F.M. Randomized phase III trial of pegylated liposomal doxorubicin versus vinorelbine or mitomycin C plus vinblastine in women with taxane-refractory advanced breast cancer. Breast Dis. a YB Q. 2005, 16, 186–187. [Google Scholar] [CrossRef]



| Carriers for Drug Delivery | Advantages | Disadvantages | References |
|---|---|---|---|
| Polymeric nanoparticles |
|
| [82,83,84,85,86] |
| Inorganic nanoparticles |
|
| [87,88,89] |
| Nanolipids |
|
| [83,90,91,92,93] |
| Hydrogels |
|
| [94,95] |
| Study | Outcomes | Material | Anticancer Drug/Small Molecules | Target Cells/Target Tumor | Delivery Approach | Type of Study | Sex/Species | Reference |
|---|---|---|---|---|---|---|---|---|
| Microbranchytherapy for intratumoral injection of holmium-166 microspheres into 13 cats with inoperable OSCC |
| PLA microspheres loaded with holmium acetylacetonate and then suspended in Pluronic F-68 solution | Holmium-166 microspheres | Tumors located in the: tongue/sublingual (n = 10); gingiva of the mandible (n = 1); gingiva or the maxilla (n = 2) | Intratumoral injection of radioactive agents | In vivo | Eight male and five female cats | [165] |
| Injection of drug loaded gels into tumors (up to 6 weeks treatments), at dosage: 0.25 mL of active or placebo gel per cm3 of the tumor up to 10 mL total |
| Purified bovine collagen/gel | Cisplatin/Epinephrine | Head and neck tumors | Intratumoral | Clinical study (178 patients pretreated with recurrent or refractory HNSCC); prospective, double-blind placebo-controlled phase III trials | Male and female humans | [147] |
| SAHA and DDP were loaded into a biodegradable and thermosensitive hydrogel (PECE) |
| PECE | Cisplatin (DDP)/SAHA | In vitro: HSC-3 and HOK16-E6E7 cells. In vivo: 2 × 106 HSC-3 cells were injected subcutaneously into the right flank regions | Intratumoral | In vitro and in vivo | Female mice | [137] |
| Synthesizing DTX encapsulated PLGA nanoparticles for in situ delivery to the tumor site |
| PLGA | Docetaxel (DTX) | Human tongue squamous carcinoma derived cell line SCC-9 | Intratumoral | In vitro | N/A | [166] |
| Irradiation following intra-tumoral injection of gold nanorods (GNRs) conjugated with rose bengal (RB) |
| Gold nanorods (GNRs)/Rose Bengal | - | Tumors induced in hamster cheek pouches | Intratumoral combined with photo-dynamic (PDT) and photothermal (PTT) therapy | In vitro and in vivo | Male hamsters | [167] |
| Synthesizing and drug encapsulation of EA loaded chitosan nanoparticles |
| Chitosan | Ellagic acid (EA) | Human oral cancer KB cell line | local | In vitro | N/A | [108] |
| Curcumin-loaded in PCL nanoparticles and coated with chitosan as a mucoadhesive polymer |
| Chitosan | Curcumin | SCC-9 human oral squamous carcinoma cell; for permeation studies: esophageal mucosa of at least two different animals | local | In vitro | N/A | [104] |
| Nano-emulsions loaded with Gen and coated with chitosan in the form of tablets |
| Nanoemulsion, chitosan, cellulose microcrystalline, dextrose | Genistein (Gen) | SCC-4 cells, FaDu cells, and murine connective tissue fibroblasts (L929) (in vitro)/ porcine buccal Mucosa (ex vivo) | local | In vitro and ex vivo | N/A | [168] |
| Using MTX loaded liposomes to prepare the mucoadhesive film |
| Liposomes, chitosan (CH), poly(vinyl alcohol) (PVA), hydroxypropyl methylcellulose (HPMC) | Methotrexate (MTX) | HSC-3 cells | local | In vitro | N/A | [169] |
| Preparation of a targeted nanoparticle platform combing Pc 4 with IO and a cancer targeting ligand, then intravenous injection of non-formulated Pc4 and two nanoparticle formulations: targeted (Fmp-IO-Pc4) and non-targeted (IO-Pc4) were administered to mice |
| Iron oxide (IO) nanoparticles | PDT drug (Pc 4) | In vitro: M4E, M4E-15, 686LN, and TU212 cell lines | PDT | In vitro and in vivo | Female mice | [170] |
| Preparation of gold nanoparticles conjugated with anti-EGFR antibody, then evaluation of the effect of PDT combined with administration of anti-EGFR antibody conjugated Au nanoparticles on two OSCC lines and one epithelial cell line |
| Anti-EGFR antibody conjugated gold nanoparticles | - | Two OSCC cell lines (HSC 313 and HOC 3 Clone 8 ); one benign epithelial cell line (HaCaT) | PDT | In vitro | N/A | [110] |
| Preparation of self-assembled core-shell nanoparticles loaded with cisplatin and pyrolipid for treatment of resistant head and neck cancers. |
| 1,2-dioleoyl-sn-glycero-3- phosphate sodium salt (DOPA) coated nanoscale coordination polymer (NCP)-based core-shell Nanoparticles with PEG | Cisplatin and pyrolipid (as photosensitizer) | In vitro: cisplatin-sensitive HNSCC135 and SCC61 as well as cisplatin-resistant JSQ3 and SQ20B In vivo: SQ20B subcutaneous xenograft murine models | PDT | In vitro and in vivo | Female Mice | [158] |
| Injection of anti-EGFR-microbubbles into the tumor site, with intravenous injection of BLM 5 min after microbubble injection |
| Liposomes with PEG chains | Bleomycin (BLM) | In vitro: Ca9-22 cells In vivo: Ca9-22 cells injected into the back of mice | Local using microbubbles and ultrasound | In vitro and in vivo | Male Mice | [42] |
| Sonoporation using microbubbles with anti-EGFR antibody and administration of BLM to assess its effect on Ca9-22 growth |
| SonoVue as microbubble agent | BLM | Ca9-22 cell line | Local using microbubbles and ultrasound | In vitro | N/A | [43] |
| Drugs | Mechanism of Action | Reference |
|---|---|---|
| Cetuximab, panitumumab, zalutumumab and nimotuzumab | EGFR inhibitors | [171] |
| Gefitinib, erlotinib, lapatinib, afatinib and dacomitinib | EGFR tyrosine kinase inhibitors | [171] |
| Bevacizumab | VEGF inhibitors | [171] |
| Sorafenib, sunitinib and vandetanib | VEGFR inhibitors | [171] |
| Rapamycin, temsirolimus, everolimus, torin1, PP242 and PP30, BYL719 | PI3K/AKT/mTOR pathway inhibitors | [171,172] |
| Pembrolizumab and nivolumab | Anti-PD-1 antibodies | [171] |
| Motolimond ( VTX-2337) | TLR8 agonist | [173] |
| AZD1775 (Adavosertib) | Elective small molecule inhibitor of WEE1 G2 checkpoint serin/threoin/protein kinase | [174] |
| Abemaciclib ( LY2835219) | Cyclin-dependent kinase inhibitor | [175] |
| TPST-1120 | Selective antagonist of PPARα | [176] |
| Sitravatinib (MGCD516) | RTK inhibitor | [177] |
| Nintedanib (BIBF1120) | Triple receptor tyrosine kinase inhibitor (PDGFR/FGFR and VEGFR) | [178] |
| Durvalumab (Imfinzi, MEDI4736) | (IgG1κ) monoclonal antibody | [179,180] |
| Tremelimumab | Anti-CTLA4 antibody | [170,181] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ketabat, F.; Pundir, M.; Mohabatpour, F.; Lobanova, L.; Koutsopoulos, S.; Hadjiiski, L.; Chen, X.; Papagerakis, P.; Papagerakis, S. Controlled Drug Delivery Systems for Oral Cancer Treatment—Current Status and Future Perspectives. Pharmaceutics 2019, 11, 302. https://doi.org/10.3390/pharmaceutics11070302
Ketabat F, Pundir M, Mohabatpour F, Lobanova L, Koutsopoulos S, Hadjiiski L, Chen X, Papagerakis P, Papagerakis S. Controlled Drug Delivery Systems for Oral Cancer Treatment—Current Status and Future Perspectives. Pharmaceutics. 2019; 11(7):302. https://doi.org/10.3390/pharmaceutics11070302
Chicago/Turabian StyleKetabat, Farinaz, Meenakshi Pundir, Fatemeh Mohabatpour, Liubov Lobanova, Sotirios Koutsopoulos, Lubomir Hadjiiski, Xiongbiao Chen, Petros Papagerakis, and Silvana Papagerakis. 2019. "Controlled Drug Delivery Systems for Oral Cancer Treatment—Current Status and Future Perspectives" Pharmaceutics 11, no. 7: 302. https://doi.org/10.3390/pharmaceutics11070302
APA StyleKetabat, F., Pundir, M., Mohabatpour, F., Lobanova, L., Koutsopoulos, S., Hadjiiski, L., Chen, X., Papagerakis, P., & Papagerakis, S. (2019). Controlled Drug Delivery Systems for Oral Cancer Treatment—Current Status and Future Perspectives. Pharmaceutics, 11(7), 302. https://doi.org/10.3390/pharmaceutics11070302