Entrapment of N-Hydroxyphthalimide Carbon Dots in Different Topical Gel Formulations: New Composites with Anticancer Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Gel Formulations Based on CARB, AS, and CMC
2.2.2. Characterization of the Prepared NHF Carbon Dots
2.2.3. Rheological Studies
2.2.4. Fluorescence Analysis
2.2.5. Cell Culture
2.2.6. Cell Proliferation and Apoptosis Activity
2.2.7. Mitochondrial Activity
2.2.8. 3D Matrigel Assays
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Gel Formulations
3.2. Rheology Studies
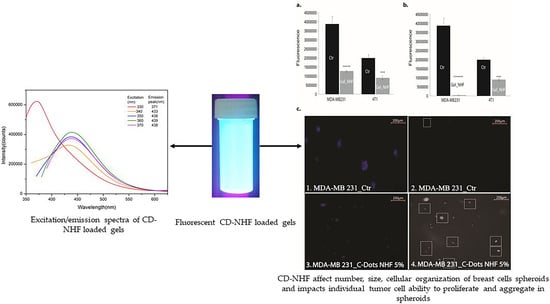
3.3. Fluorescence Analysis
3.4. In Vitro Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A375 | human malignant melanoma |
| AS | sodium alginate |
| B16F10 | mouse malignant melanoma cells |
| Balb/c-5064 | mouse dermal microvascular endothelial cells |
| CARB | Ultrez 10 carbomer |
| CD | carbon dots |
| CD-NHF | N-hydroxyphthalimide carbon dots |
| Conc. | concentration |
| CMC | carboxymethyl cellulose |
| CR | controlled strain mode |
| CS | controlled stress mode |
| FBS | fetal bovine serum |
| GLY | glycerin |
| HDMVECn | primary dermal microvascular endothelial cells |
| MDA-MB-231 | human mammary breast cancer |
| PBS | phosphate-buffered saline |
| λem | emission maximum |
| λex | excitation wavelength |
References
- Vishnubhakthula, S.; Elupula, R.; Durán-Lara, E.F. Recent advances in hydrogel-based drug delivery for melanoma cancer therapy: A mini review. J. Drug Deliv. 2017, 2017, 7275985. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, P.; Tatke, P. Design of an encapsulated topical formulation for chemoprevention of skin cancer. Int. J. Pharm. Sci. Res. 2019, 10, 309–319. [Google Scholar] [CrossRef]
- Sun, X.; Li, G.; Yin, Y.; Zhang, Y.; Li, H. Carbon quantum dot-based fluorescent vesicles and chiral hydrogels with biosurfactant and biocompatible small molecule. Soft Matter. 2018, 14, 6983–6993. [Google Scholar] [CrossRef] [PubMed]
- Stan, C.S.; Gospei Horlescu, P.; Ursu, L.E.; Popa, M.; Albu, C. Facile preparation of highly luminescent composites by polymer embedding of carbon dots derived from N-hydroxyphthalimide. J. Mater. Sci. 2017, 52, 185–196. [Google Scholar] [CrossRef]
- Peng, Z.; Miyanji, E.H.; Zhou, Y.; Pardo, J.; Hettiarachchi, S.D.; Li, S.; Blackwelder, P.L.; Skromne, I.; Leblanc, R.M. Carbon dots: Promising biomaterials for bone-specific imaging and drug delivery. Nanoscale 2017, 9, 17533–17543. [Google Scholar] [CrossRef] [PubMed]
- Vasimalai, N.; Vilas-Boas, V.; Gallo, J.; Cerqueira, M.F.; Menéndez-Miranda, M.; Costa-Fernández, J.M.; Fernández-Argüelles, M.T. Green synthesis of fluorescent carbon dots from spices for in vitro imaging and tumour cell growth inhibition. Beilstein J. Nanotechnol. 2018, 9, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Djabourov, M. Gelation of physical gels: The gelatin gels. In Physics of Finely Divided Matter; Boccara, N., Daoud, M., Eds.; Springer: Berlin, Germany, 1985; pp. 21–23. [Google Scholar]
- Desbrieres, J.; Peptu, C.A.; Savin, C.-L.; Popa, M. Chemically modified polysaccharides with applications in nanomedicine. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value, 1st ed.; Popa, V., Volf, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 351–399. [Google Scholar] [CrossRef]
- Xu, H.; Xu, X. Polysaccharide, a potential anti-cancer drug with high efficacy and safety. Adv. Oncol. Res. Treat 2016, 2, 110. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural polymers. Natural and synthetic biomedical polymers. In Natural and Synthetic Biomedical Polymers, 1st ed.; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Burlington, MA, USA, 2014; pp. 67–89. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Durgadevi, R.; Saravanan, K.; Shivsankar, K.; Usha, S.; Saravanan, M. Formulation of bioadhesive carbomer gel incorporating drug-loaded gelatin microspheres for periodontal therapy. Trop. J. Pharm. Res. 2012, 11, 335–343. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, A.; An, T.; Kong, L.; Yu, C.; Liu, J.; Xia, C.; Zhou, H.; Li, Y. N-Hydroxyphthalimide exhibits antitumor activity by suppressing mTOR signaling pathway in BT-20 and LoVo cells. J. Exp. Clin. Cancer Res. 2016, 35, 41. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer 2009, 9, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Amoêdo, N.D.; Valencia, J.P.; Rodrigues, M.F.; Galina, A.; Rumjanek, F.D. How does the metabolism of tumour cells differ from that of normal cells. Biosci. Rep. 2013, 33, e00080. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oakes, S.A.; Korsmeyer, S.J. Untangling the web: Mitochondrial fission and apoptosis. Dev. Cell 2004, 7, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Grandemange, S.; Herzig, S.; Martinou, J.C. Mitochondrial dynamics and cancer. Semin. Cancer Biol. 2009, 19, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellerbach, A.; Schuster, V.; Jansen, A.; Sommer, J. MRI phantoms—Are there alternatives to agar? PLoS ONE 2013, 8, e70343. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]










| Sample Code | CARB, % | AS, % | CMC, % | GLY, % | Distilled Water (mL) | Ethanol (mL) | CD-NHF (g) |
|---|---|---|---|---|---|---|---|
| CARB-F1 | 3.8 | - | - | - | 10 | 3 | - |
| CARB-F2 | 4.6 | - | - | - | - | ||
| CARB-F3 | 5.8 | - | - | - | - | ||
| CARB-F4 | 4.6 | - | - | - | 0.050 | ||
| AS-F1 | 4.6 | 4.6 | - | - | - | ||
| AS-F2 | 4.6 | 4.6 | - | 9.2 | - | ||
| AS-F3 | 3.5 | 4.6 | - | 8.1 | - | ||
| AS-F4 | 2.3 | 4.6 | - | 6.9 | - | ||
| AS-F5 | 1.2 | 4.6 | - | 5.8 | - | ||
| AS-F6 | 4.6 | 4.6 | - | 5.8 | 0.050 | ||
| CMC-F1 | 2.9 | - | 2.9 | - | - | ||
| CMC-F2 | 2.9 | - | 2.9 | 5.8 | - | ||
| CMC-F3 | 3.6 | - | 2.9 | 6.5 | - | ||
| CMC-F4 | 4.3 | - | 2.9 | 7.2 | - | ||
| CMC-F5 | 5.8 | - | 2.9 | 8.7 | - | ||
| CMC-F6 | 3.6 | - | 2.9 | 6.5 | 0.050 |
| Sample Code | γLVE (%) |
|---|---|
| CARB-F2 | 0.25 |
| CARB-F4 | 0.25 |
| AS-F5 | 5 |
| AS-F6 | 5 |
| CMC-F3 | 10 |
| CMC-F6 | 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savin, C.-L.; Tiron, C.; Carasevici, E.; Stan, C.S.; Ibanescu, S.A.; Simionescu, B.C.; Peptu, C.A. Entrapment of N-Hydroxyphthalimide Carbon Dots in Different Topical Gel Formulations: New Composites with Anticancer Activity. Pharmaceutics 2019, 11, 303. https://doi.org/10.3390/pharmaceutics11070303
Savin C-L, Tiron C, Carasevici E, Stan CS, Ibanescu SA, Simionescu BC, Peptu CA. Entrapment of N-Hydroxyphthalimide Carbon Dots in Different Topical Gel Formulations: New Composites with Anticancer Activity. Pharmaceutics. 2019; 11(7):303. https://doi.org/10.3390/pharmaceutics11070303
Chicago/Turabian StyleSavin, Corina-Lenuta, Crina Tiron, Eugen Carasevici, Corneliu S. Stan, Sorin Alexandru Ibanescu, Bogdan C. Simionescu, and Catalina A. Peptu. 2019. "Entrapment of N-Hydroxyphthalimide Carbon Dots in Different Topical Gel Formulations: New Composites with Anticancer Activity" Pharmaceutics 11, no. 7: 303. https://doi.org/10.3390/pharmaceutics11070303
APA StyleSavin, C. -L., Tiron, C., Carasevici, E., Stan, C. S., Ibanescu, S. A., Simionescu, B. C., & Peptu, C. A. (2019). Entrapment of N-Hydroxyphthalimide Carbon Dots in Different Topical Gel Formulations: New Composites with Anticancer Activity. Pharmaceutics, 11(7), 303. https://doi.org/10.3390/pharmaceutics11070303