Patient Centric Pharmaceutical Drug Product Design—The Impact on Medication Adherence
Abstract
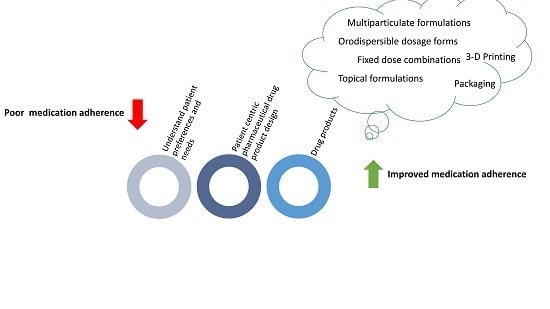
:1. Introduction
1.1. Taxonomy
- Initiation, which defines the moment that the patient takes the first dose;
- Implementation, which is the extent to which a patient’s actual dosing corresponds to the prescribed dosing regimen, from initiation until the last dose;
- Persistence, which is the length of time between initiation and the last dose.
1.2. Impact on Public Health
1.3. Determinants of Non-Adherence
2. Patient Centric Pharmaceutical Drug Product Design: Defining Target Product Profiles for Special Populations
2.1. Elderly
2.2. Paediatric Population
2.3. Dermatological Patients
3. Patient Centric Pharmaceutical Drug Product Design: The Impact on Medication Adherence
3.1. Packaging
3.2. Fixed Dose Combinations (FDCs)
3.3. Orodispersible Dosage Forms
3.4. Multi-Particulate Formulations
3.5. Topical Formulations
3.6. 3D Printing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vrijens, B.; De Geest, S.; Hughes, D.A.; Przemyslaw, K.; Demonceau, J.; Ruppar, T.; Dobbels, F.; Fargher, E.; Morrison, V.; Lewek, P.; et al. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Andrzejczyk, A.; Clyne, W.; De Geest, S.; Demonceau, J.; Dobbels, F.; Fargher, E.; Hunghes, D.; Kardas, P.; Lewek, P.; Matyjaszczyk, M.; et al. Ascertaining Barriers for Compliance: Policies for Safe, Efective and Cost-Efective Use of Medicines in Europe; ABC Project Final Report; Ascertaining Barriers for Compliance: Lodz, Poland, 2012. [Google Scholar]
- Cramer, J.A.; Roy, A.; Burrell, A.; Fairchild, C.J.; Fuldeore, M.J.; Ollendorf, D.A. Medication compliance and persistence: Terminology and deinitions. Value Health 2008, 11, 44–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugtenburg, J.G.; Timmers, L.; Elders, P.J.; Vervloet, M.; Van Dijk, L. Definitions, variants, and causes of nonadherence with medication: A challenge for tailored interventions. Patient Prefer. Adherence 2013, 7, 675–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osterberg, T.B.L. Adherence to Medication—NEJM. NEJM 2005, 353, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Tunstall-Pedoe, H. Preventing Chronic Diseases. In A Vital Investment: WHO Global Report; World Health Organization: Geneva, Switzerland, 2005; p. 200. ISBN 92 4 1563001. [Google Scholar]
- World Health Organization. Global Status Report on Noncommunicable Diseases (WHO/NMH/NVI/15.1); WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Menditto, E.; Cahir, C.; Aza-Pascual-Salcedo, M.; Bruzzese, D.; Poblador-Plou, B.; Malo, S.; Costa, E.; Rubio, F.G.; Gimeno-Miguel, A.; Orlando, V.; et al. Adherence to chronic medication in older populations: Application of a common protocol among three European cohorts. Patient Prefer. Adherence 2018, 12, 1975–1987. [Google Scholar] [CrossRef] [Green Version]
- Iolascon, G.; Gimigliano, F.; Moretti, A.; Riccio, I.; Di Gennaro, M.; Illario, M.; Monetti, V.M.; Orlando, V.; Menditto, E. Rates and reasons for lack of persistence with anti-osteoporotic drugs: Analysis of the Campania region database. Clin. Cases Miner. Bone Metab. 2016, 13, 127–130. [Google Scholar] [CrossRef]
- Iolascon, G.; Gimigliano, F.; Orlando, V.; Capaldo, A.; Di Somma, C.; Menditto, E. Osteoporosis drugs in real-world clinical practice: An analysis of persistence. Aging Clin. Exp. Res. 2013, 25, 137–141. [Google Scholar] [CrossRef]
- Casula, M.; Catapano, A.L.; Piccinelli, R.; Menditto, E.; Manzoli, L.; De Fendi, L.; Orlando, V.; Flacco, M.E.; Gambera, M.; Filippi, A.; et al. Assessment and potential determinants of compliance and persistence to antiosteoporosis therapy in Italy. Am. J. Manag. Care 2014, 20, 138–145. [Google Scholar]
- Busse, R.; Blümel, M.; Scheller-Kreinsen, D.; Zentner, A. Tackling Chronic Disease in Europe. In Strategies, Interventions and Challenges; Observatory Studies Series no. 20; European Observatory on health systems and policies: Copenhagen, Denmark, 2010. [Google Scholar]
- WHO Centre for Health Development (WHO/WKC/Tech.Ser./04.2). A Glossary of Terms for Community Health Care and Services for Older Persons; WHO Centre for Health Development: Geneva, Switzerland, 2004. [Google Scholar]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Mangin, D.; Heath, I.; Jamoulle, M. Beyond diagnosis: Rising to the multimorbidity challenge. BMJ 2012, 344, e3526. [Google Scholar] [CrossRef]
- Bogardus, S.T.; Tinetti, M.E.; Agostini, J.V. Potential Pitfalls of Disease-Specific Guidelines for Patients with Multiple Conditions. N. Engl. J. Med. 2004, 351, 2870–2874. [Google Scholar]
- IMS. Institute for Healthcare Informatics Avoidable Costs in US Health Care. 10 September 2013. Available online: http://offers.premierinc.com/rs/381-NBB-525/images/Avoidable_Costs_in%20_US_Healthcare-IHII_AvoidableCosts_2013%5B1%5D.pdf (accessed on 1 October 2019).
- Sokol, M.C.; McGuigan, K.A.; Verbrugge, R.R.; Epstein, R.S. Impact of medication adherence on hospitalization risk and healthcare cost: Discovery Service for Tartu University. Med. Care 2005, 43, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Roebuck, M.C.; Liberman, J.N.; Gemmill-Toyama, M.; Brennan, T.A. Medication Adherence Leads to Lower Health Care Use And Costs Despite Increased Drug Spending. Health Aff. 2011, 30, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putignano, D.; Bruzzese, D.; Orlando, V.; Fiorentino, D.; Tettamanti, A.; Menditto, E. Differences in drug use between men and women: An Italian cross sectional study. BMC Women’s Health 2017, 17, 73. [Google Scholar] [CrossRef] [Green Version]
- De Geest, S.; Sabaté, E.; Sabat, E. Adherence to Long-Term Therapies: Evidence for Action. Eur. J. Cardiovasc. Nurs. 2003, 2, 323. [Google Scholar] [CrossRef]
- Wilke, T.; Müller, S.; Morisky, N.E. Toward Identifying the Causes and Combinations of Causes Increasing the Risks of Nonadherence to Medical Regimens: Combined Results of Two German Self-Report Surveys. Value Health 2011, 14, 1092–1100. [Google Scholar] [CrossRef] [Green Version]
- Kardas, P.; Lewek, P.; Matyjaszczyk, M. Determinants of patient adherence: A review of systematic reviews. Front. Pharmacol. 2013, 4, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Scala, D.; Menditto, E.; Armellino, M.F.; Manguso, F.; Monetti, V.M.; Orlando, V.; Antonino, A.; Makoul, G.; De Palma, M. Italian translation and cultural adaptation of the communication assessment tool in an outpatient surgical clinic. BMC Health Serv. Res. 2016, 16, 163. [Google Scholar] [CrossRef] [Green Version]
- Yap, A.F.; Thirumoorthy, T.; Kwan, Y.H. Systematic review of the barriers affecting medication adherence in older populations. Geriatr. Gerontol. Int. 2016, 16, 1093–1101. [Google Scholar] [CrossRef]
- McLoughlin, A.; Bennett, K.; Cahir, C. Developing a model of the determinants of medication nonadherence in older community-dwelling patients. Ann. Behav. Med. 2019, 53, 942–954. [Google Scholar] [CrossRef]
- Phatak, H.; Joseph, T., 3rd. Relationships between beliefs about medication and nonadherence to prescribed chronic medications. Ann. Pharmacother. 2006, 40, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Giardini, A.; Martin, M.T.; Cahir, C.; Lehane, E.; Menditto, E.; Strano, M.; Pecorelli, S.; Monaco, A.; Marengoni, A. Toward appropriate criteria in medication adherence assessment in older persons: Position Paper. Aging Clin. Exp. Res. 2016, 28, 371–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malo, S.; Kardas, P.; Menditto, E. Some reflections concerning the assessment of patient adherence and persistence to medication. Curr. Med. Res. Opin. 2018, 35, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, S.; Ternik, R.L.; Onder, G.; Khan, M.A.; Van Riet-Nales, D.A. Defining Patient Centric Pharmaceutical Drug Product Design. AAPS J. 2016, 18, 1047–1055. [Google Scholar] [CrossRef] [Green Version]
- Stegemann, S. Patient centric drug product design in modern drug delivery as an opportunity to increase safety and effectiveness. Expert Opin. Drug Deliv. 2018, 15, 619–627. [Google Scholar] [CrossRef]
- EMA. Reflection Paper on the Pharmaceutical Development of Medicines for Use in the Older Population (EMA/CHMP/QWP/292439). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-pharmaceutical-development-medicines-use-older-population-first-version_en.pdf (accessed on 4 September 2019).
- Gnjidic, D.; Husband, A.; Todd, A. Challenges and innovations of delivering medicines to older adults. Adv. Drug Deliv. Rev. 2018, 135, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Sultana, J.; Musazzi, U.M.; Ingrasciotta, Y.; Giorgianni, F.; Ientile, V.; Fontana, A.; Minghetti, P.; Perrotta, M.; Santoro, D.; Savica, V.; et al. Medication is an additional source of phosphate intake in chronic kidney disease patients. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 959–967. [Google Scholar] [CrossRef]
- EMA. Reflection Paper on Physical Frailty: Instruments for Baseline Characterisation of Older Populations in Clinical Trials (EMA/CHMP/778709/2015); EMA: London, UK, 2018. [Google Scholar]
- EMEA. Adequacy of Guidance on the Elderly Regarding Medicinal Products for Human Use (EMEA/498920/2006); EMEA: London, UK, 2006. [Google Scholar]
- Marquis, J.; Schneider, M.-P.; Payot, V.; Cordonier, A.-C.; Bugnon, O.; Hersberger, K.E.; Arnet, I. Swallowing difficulties with oral drugs among polypharmacy patients attending community pharmacies. Int. J. Clin. Pharm. 2013, 35, 1130–1136. [Google Scholar] [CrossRef]
- EMA. Concept Paper on the Need for a Reflection Paper on Quality Aspects of Medicines for Older People (EMA/165974/2013); EMA: London, UK, 2013. [Google Scholar]
- Mosca, C.; Castel-Branco, M.M.; Ribeiro-Rama, A.C.; Caramona, M.M.; Fernandez-Llimos, F.; Figueiredo, I.V. Assessing the impact of multi-compartment compliance aids on clinical outcomes in the elderly: A pilot study. Int. J. Clin. Pharm. 2014, 36, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Conn, V.S.; Ruppar, T.M.; Chan, K.C.; Dunbar-Jacob, J.; Pepper, G.A.; De Geest, S. Packaging interventions to increase medication adherence: Systematic review and meta-analysis. Curr. Med. Res. Opin. 2015, 31, 145–160. [Google Scholar] [CrossRef]
- EMA. Good Practice Guide Medication Error risk Minimisation and Prevention (EMA/606103/2014); EMA: London, UK, 2015. [Google Scholar]
- Chubaty, A.; Sadowski, C.A.; Carrie, A.G. Typeface legibility of patient information leaflets intended for community-dwelling seniors. Age Ageing 2009, 38, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, F.; Chernew, M.E.; Fendrick, A.M. Impact of Fixed-Dose Combination Drugs on Adherence to Prescription Medications. J. Gen. Intern. Med. 2008, 23, 611–614. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and the Council. Regulation No 1901/2006 on Medicinal Products for Paediatric Use and Amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC), (2006); European Parliament and the Council: Brussels, Belgium, 2006. [Google Scholar]
- EMA. Guideline on Pharmaceutical Development of Medicines for Paediatric Use Guideline on Pharmaceutical Development of Medicines for Paediatric Use (EMA/CHMP/QWP/805880/2012 Rev.2); EMA: London, UK, 2013. [Google Scholar]
- EMA. ICH E11(R1) Guideline on Clinical Investigation of Medicinal Products in the Pediatric Population (EMA/CPMP/ICH/2711/1999); EMA: London, UK, 2016. [Google Scholar]
- EMA. Reflection Paper on the Use of Extrapolation in the Development of Medicines for Paediatrics (EMA/189724/2018); EMA: London, UK, 2018. [Google Scholar]
- Kliegman, R.M.; St. Geme, J.W.; Blum, N.J.; Shah, S.S.; Tasker, R.C. Nelson Textbook of Pediatrics, 21st ed.; Elsevier: Philadelphia, PA, USA, 2019. [Google Scholar]
- Le Révérend, B.J.D.; Edelson, L.R.; Loret, C. Anatomical, functional, physiological and behavioural aspects of the development of mastication in early childhood. Br. J. Nutr. 2014, 111, 403–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Riet-Nales, D.A.; Schobben, A.F.A.M.; Vromans, H.; Egberts, T.C.G.; Rademaker, C.M.A. Safe and effective pharmacotherapy in infants and preschool children: Importance of formulation aspects. Arch. Dis. Child. 2016, 101, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Ranmal, S.R.; Cram, A.; Tuleu, C. Age-appropriate and acceptable paediatric dosage forms: Insights into end-user perceptions, preferences and practices from the Children’s Acceptability of Oral Formulations (CALF) Study. Int. J. Pharm. 2016, 514, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Kardas, P.; Muras, M. A blinded comparison of palatability of 13 common pediatric antibiotic suspensions. Wiad. Lek. 2005, 58, 15–20. [Google Scholar]
- Pein, M.; Preis, M.; Eckert, C.; Kiene, F.E. Taste-masking assessment of solid oral dosage forms—A critical review. Int. J. Pharm. 2014, 465, 239–254. [Google Scholar] [CrossRef]
- Mistry, P.; Batchelor, H. Evidence of acceptability of oral paediatric medicines: A review. J. Pharm. Pharmacol. 2017, 69, 361–376. [Google Scholar] [CrossRef] [Green Version]
- EMA. Reflection Paper: Formulation of Choice for Paediatric Population (EMEA/CHMP/PEG/194810/2005); EMA: London, UK, July 2006. [Google Scholar]
- Neuspiel, D.R.; Taylor, M.M. Reducing the Risk of Harm from Medication Errors in Children. Health Serv. Insights 2013, 6, 47–59. [Google Scholar] [CrossRef]
- Casiraghi, A.; Musazzi, U.M.; Franceschini, I.; Berti, I.; Paragò, V.; Cardosi, L.; Minghetti, P. Is propranolol compounding from tablet safe for pediatric use? Results from an experimental test. Minerva Pediatr. 2014, 66, 355–362. [Google Scholar]
- Somogyi, O.; Meskó, A.; Csorba, L.; Szabó, P.; Zelkó, R. Pharmaceutical counselling about different types of tablet-splitting methods based on the results of weighing tests and mechanical development of splitting devices. Eur. J. Pharm. Sci. 2017, 106, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, P.; Pantano, D.; Gennari, C.G.M.; Casiraghi, A. Regulatory framework of pharmaceutical compounding and actual developments of legislation in Europe. Health Policy 2014, 117, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Svensson, Å.; Ofenloch, R.; Bruze, M.; Naldi, L.; Cazzaniga, S.; Elsner, P.; Gonçalo, M.; Schuttelaar, M.-L.; Diepgen, T. Prevalence of skin disease in a population-based sample of adults from five European countries. Br. J. Dermatol. 2018, 178, e368. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.; Gaio, A.R.; Lobo, J.M.S.; De Almeida, I.F.M.; Teixeira, A. Development and Validation of a Novel Questionnaire for Adherence with Topical Treatments in Psoriasis (QATOP). Am. J. Clin. Dermatol. 2017, 18, 571–581. [Google Scholar]
- Krejci-Manwaring, J.; Mccarty, M.A.; Camacho, F.; Carroll, C.L.; Johnson, K.; Manuel, J.; Balkrishnan, R.; Hartle, J.; Fleischer, A.; Feldman, S.R. Adherence with topical treatment is poor compared with adherence with oral agents: Implications for effective clinical use of topical agents. J. Am. Acad. Dermatol. 2006, 54, S235–S236. [Google Scholar] [CrossRef]
- Eastman, W.J.; Malahias, S.; Delconte, J.; DiBenedetti, D. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis 2014, 94, 46–53. [Google Scholar]
- Vasconcelos, V.; Teixeira, A.; Almeida, V.; Teixeira, M.; Ramos, S.; Torres, T.; Lobo, J.M.S.; Almeida, I.F. Patient preferences for attributes of topical anti-psoriatic medicines. J. Dermatol. Treat. 2018, 30, 659–663. [Google Scholar] [CrossRef]
- Drumond, N.; Van Riet-Nales, D.A.; Karapinar-Çarkit, F.; Stegemann, S. Patients’ appropriateness, acceptability, usability and preferences for pharmaceutical preparations: Results from a literature review on clinical evidence. Int. J. Pharm. 2017, 521, 294–305. [Google Scholar] [CrossRef]
- Ibrahim, I.R.; Ibrahim, M.I.M.; Al-Haddad, M.S. The influence of consumers’ preferences and perceptions of oral solid dosage forms on their treatment. Int. J. Clin. Pharm. 2012, 34, 728–732. [Google Scholar] [CrossRef]
- Overgaard, A.; Møller-Sonnergaard, J.; Christrup, L.; Højsted, J.; Hansen, R. Patients’ evaluation of shape, size and colour of solid dosage forms. Pharm. World Sci. 2001, 23, 185–188. [Google Scholar] [CrossRef]
- Stolk, G.; Kwint, H.-F.; Faber, A.; Gussekloo, J.; Bouvy, M.L. Medication adherence and knowledge of older patients with and without multidose drug dispensing. Age Ageing 2013, 42, 620–626. [Google Scholar]
- Zedler, B.K.; Kakad, P.; Colilla, S.; Murrelle, L.; Shah, N.R. Does Packaging with a Calendar Feature Improve Adherence to Self-Administered Medication for Long-Term Use? A Systematic Review. Clin. Ther. 2011, 33, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Galdo, J.; Cox, E.D.; Moreno, M.A.; Young, H.N. Impact of Bubble Packaging on Adherence to Long-Term Oral Medications Used to Prevent Cardiovascular Disease. J. Pharm. Technol. 2017, 33, 114–120. [Google Scholar] [CrossRef]
- van Rein, N.; de Geus, K.S.; Cannegieter, S.C.; Reitsma, P.H.; van der, F.J.M.; Lijfering, W.M. Multi-dose drug dispensing as a tool to improve medication adherence: A study in patients using vitamin K antagonists. Pharmacoepidemiol. Drug Saf. 2018, 27, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Kamalakkannan, G.; Parkar, S.; Messerli, F.H. Fixed-Dose Combinations Improve Medication Compliance: A Meta-Analysis. Am. J. Med. 2007, 120, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Du, L.-P.; Cheng, Z.-W.; Zhang, Y.-X.; Li, Y.; Mei, D. The impact of fixed-dose combination versus free-equivalent combination therapies on adherence for hypertension: A meta-analysis. J. Clin. Hypertens. 2018, 20, 902–907. [Google Scholar] [CrossRef] [Green Version]
- EMA. Guideline on Clinical Development of Fixed Combination Medicinal Products End of Consultation (Deadline for Comments) GUIDELINE on Clinical Development of Fixed Combination Medicinal Products; EMA: London, UK, March 2017; p. 44. [Google Scholar]
- Webster, R.; Rodgers, A. Polypill treatments for cardiovascular diseases. Expert Opin. Drug Deliv. 2016, 13, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Blonde, L.; Juan, Z.T.S.; Bolton, P. Fixed-Dose Combination Therapy in Type 2 Diabetes Mellitus. Endocr. Pract. 2014, 1, 1–32. [Google Scholar] [CrossRef]
- Putignano, D.; Orlando, V.; Monetti, V.M.; Piccinocchi, G.; Musazzi, U.M.; Piccinocchi, R.; Minghetti, P.; Menditto, E. Fixed versus Free Combinations of Antihypertensive Drugs: Analyses of Real-World Data of Persistence with Therapy in Italy. Patient Prefer. Adherence 2019, 13, 1961–1969. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Panjabi, S.; Sherrill, B.; Halpern, M.; Zhang, J. Single-Pill vs Free-Equivalent Combination Therapies for Hypertension: A Meta-Analysis of Health Care Costs and Adherence. J. Clin. Hypertens. 2011, 13, 898–909. [Google Scholar]
- Teo, K.; Yusuf, S. Polypill Variants (Quarter Pill Trials). Am. J. Hypertens. 2018, 31, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Blanco, J.L.; Sánchez-Palomino, S.; Marcos, M.A.; Guardo, A.C.; González-Cordón, A.; Lonca, M.; Tricas, A.; Rodriguez, A.; Romero, A.; et al. A maintenance 3-day-per-week schedule with the single tablet regimen efavirenz/emtricitabine/tenofovir disoproxil fumarate is effective and decreases sub-clinical toxicity. AIDS 2018, 32, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Mancia, G. Hypertension: Quarter dose quadpill combinations: A new therapeutic approach. Nat. Rev. Nephrol. 2017, 13, 266–267. [Google Scholar] [CrossRef]
- Bennett, A.; Chow, C.K.; Chou, M.; Dehbi, H.M.; Webster, R.; Salam, A.; Patel, A.; Neal, B.; Peiris, D.; Thakkar, J.; et al. Efficacy and Safety of Quarter-Dose Blood Pressure-Lowering Agents: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Hypertension 2017, 70, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Saravolatz, L.D.; Johnson, L.B. The quad pill, a once-daily combination therapy for HIV infection. Clin. Infect. Dis. 2014, 58, 93–98. [Google Scholar]
- Chow, C.K.; Thakkar, J.; Bennett, A.; Hillis, G.; Burke, M.; Usherwood, T.; Vo, K.; Rogers, K.; Atkins, E.; Webster, R.; et al. Quarter-dose quadruple combination therapy for initial treatment of hypertension: Placebo-controlled, crossover, randomised trial and systematic review. Lancet 2017, 389, 1035–1042. [Google Scholar] [CrossRef]
- Koo, O. Manufacturing process considerations for fixed-dose combination drug products. Am. Pharm. Rev. 2010, 13, 71–75. [Google Scholar]
- Kavanagh, O.N.; Albadarin, A.B.; Croker, D.M.; Healy, A.M.; Walker, G.M. Maximising success in multidrug formulation development: A review. J. Control. Release 2018, 283, 1–19. [Google Scholar] [CrossRef]
- Desai, D.; Wang, J.; Wen, H.; Li, X.; Timmins, P. Formulation design, challenges, and development considerations for fixed dose combination (FDC) of oral solid dosage forms. Pharm. Dev. Technol. 2013, 18, 1265–1276. [Google Scholar] [CrossRef]
- Hanning, S.M.; Lopez, F.L.; Wong, I.C.; Ernest, T.B.; Tuleu, C.; Gul, M.O. Patient centric formulations for paediatrics and geriatrics: Similarities and differences. Int. J. Pharm. 2016, 512, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ming Lim, L.; Dong, B.; Hadinoto, K. Proof-of-concept preparation and characterization of dual-drug amorphous nanoparticle complex as fixed-dose combination of poorly soluble drugs. Drug Dev. Ind. Pharm. 2019, 45, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Ranmal, S.R.; Ernest, T.B.; Liu, F. Patient acceptability, safety and access: A balancing act for selecting age-appropriate oral dosage forms for paediatric and geriatric populations. Int. J. Pharm. 2018, 536, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Cilurzo, F.; Musazzi, U.M.; Franzé, S.; Selmin, F.; Minghetti, P. Orodispersible dosage forms: Biopharmaceutical improvements and regulatory requirements. Drug Discov. Today 2018, 23, 251–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tablets Monograph (01/2014:0478). European Pharmacopoeia, 9th ed.; European Directorate for the Quality of Medicines: Strasbourg, France, 2017. [Google Scholar]
- Franceschini, I.; Selmin, F.; Pagani, S.; Minghetti, P.; Cilurzo, F. Nanofiller for the mechanical reinforcement of maltodextrins orodispersible films. Carbohydr. Polym. 2016, 136, 676–681. [Google Scholar] [CrossRef]
- Selmin, F.; Franceschini, I.; Cupone, I.E.; Minghetti, P.; Cilurzo, F. Aminoacids as non-traditional plasticizers of maltodextrins fast-dissolving films. Carbohydr. Polym. 2015, 115, 613–616. [Google Scholar] [CrossRef]
- Seager, H. Drug-delivery products and the Zydis fast-dissolving dosage form. J. Pharm. Pharmacol. 1998, 50, 375–382. [Google Scholar] [CrossRef]
- Regan, J.; Sowman, R.; Walsh, I. Prevalence of Dysphagia in Acute and Community Mental Health Settings. Dysphagia 2006, 21, 95–101. [Google Scholar] [CrossRef]
- Liu, F.; Ghaffur, A.; Bains, J.; Hamdy, S. Acceptability of oral solid medicines in older adults with and without dysphagia: A nested pilot validation questionnaire based observational study. Int. J. Pharm. 2016, 512, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Bitter, I.; Treuer, T.; Dilbaz, N.; Oyffe, I.; Ciorabai, E.M.; Gonzalez, S.L.; Ruschel, S.; Salburg, J.; Dyachkova, Y. Patients’ preference for olanzapine orodispersible tablet compared with conventional oral tablet in a multinational, randomized, crossover study. World J. Boil. Psychiatry 2010, 11, 894–903. [Google Scholar] [CrossRef]
- Montgomery, W.; Treuer, T.; Karagianis, J.; Ascher-Svanum, H.; Harrison, G. Orally disintegrating olanzapine review: Effectiveness, patient preference, adherence, and other properties. Patient Prefer. Adherence 2012, 6, 109–125. [Google Scholar] [CrossRef] [Green Version]
- Mitra, B.; Chang, J.; Wu, S.-J.; Wolfe, C.N.; Ternik, R.L.; Gunter, T.Z.; Victor, M.C. Feasibility of mini-tablets as a flexible drug delivery tool. Int. J. Pharm. 2017, 525, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Klingmann, V. Acceptability of Mini-Tablets in Young Children: Results from Three Prospective Cross-over Studies. AAPS PharmSciTech 2017, 18, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Klingmann, V.; Spomer, N.; Lerch, C.; Stoltenberg, I.; Frömke, C.; Bosse, H.M.; Breitkreutz, J.; Meissner, T. Favorable Acceptance of Mini-Tablets Compared with Syrup: A Randomized Controlled Trial in Infants and Preschool Children. J. Pediatr. 2013, 163, 1728–17320. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Vasconcelos, V.; Teixeira, M.; Almeida, V.; Azevedo, R.; Torres, T.; Lobo, J.M.S.; Costa, P.C.; Almeida, I.F. Mechanical Properties of Topical Anti-Psoriatic Medicines: Implications for Patient Satisfaction with Treatment. AAPS PharmSciTech 2019, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Veverka, K.A.; Lu, M.; Armstrong, A.W. Real-world experience of calcipotriene and betamethasone dipropionate foam 0.005%/0.064% in the treatment of adults with psoriasis in the United States. J. Dermatol. Treat. 2019, 30, 454–460. [Google Scholar] [CrossRef]
- Puig, L.; Carrascosa, J.M.; Belinchón, I.; Fernández-Redondo, V.; Carretero, G.; Ruiz-Carrascosa, J.C.; Careaga, J.M.; de la Cueva, P.; Gárate, M.T.; Ribera, M.; et al. Adherence and patient satisfaction with topical treatment in psoriasis, and the use, and organoleptic properties of such treatments: A Delphi study with an expert panel and members of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology. Actas Dermosifiliogr. 2013, 104, 488–496. [Google Scholar]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. An Overview of 3D Printing Technologies for Soft Materials and Potential Opportunities for Lipid-based Drug Delivery Systems. Pharm. Res. 2018, 36, 4. [Google Scholar] [CrossRef] [Green Version]
- Scarpa, M.; Stegemann, S.; Hsiao, W.-K.; Pichler, H.; Gaisford, S.; Bresciani, M.; Paudel, A.; Orlu, M. Orodispersible films: Towards drug delivery in special populations. Int. J. Pharm. 2017, 523, 327–335. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Selmin, F.; Ortenzi, M.A.; Mohammed, G.K.; Franzé, S.; Minghetti, P.; Cilurzo, F. Personalized orodispersible films by hot melt ram extrusion 3D printing. Int. J. Pharm. 2018, 551, 52–59. [Google Scholar] [CrossRef]
- AlOmari, M.; Vuddanda, P.R.; Trenfield, S.J.; Dodoo, C.C.; Velaga, S.; Basit, A.W.; Gaisford, S. Printing T3 and T4 oral drug combinations as a novel strategy for hypothyroidism. Int. J. Pharm. 2018, 549, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Thabet, Y.; Lunter, D.; Breitkreutz, J. Continuous inkjet printing of enalapril maleate onto orodispersible film formulations. Int. J. Pharm. 2018, 546, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Sadia, M.; Isreb, A.; Abbadi, I.; Isreb, M.; Aziz, D.; Selo, A.; Timmins, P.; Alhnan, M.A. From ‘fixed dose combinations’ to ‘a dynamic dose combiner’: 3D printed bi-layer antihypertensive tablets. Eur. J. Pharm. Sci. 2018, 123, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Varan, C.; Wickström, H.; Sandler, N.; Aktaş, Y.; Bilensoy, E. Inkjet printing of antiviral PCL nanoparticles and anticancer cyclodextrin inclusion complexes on bioadhesive film for cervical administration. Int. J. Pharm. 2017, 531, 701–713. [Google Scholar] [CrossRef]
- Thabet, Y.; Sibanc, R.; Breitkreutz, J. Printing pharmaceuticals by inkjet technology: Proof of concept for stand-alone and continuous in-line printing on orodispersible films. J. Manuf. Process. 2018, 35, 205–215. [Google Scholar] [CrossRef]
- Edinger, M.; Bar-Shalom, D.; Sandler, N.; Rantanen, J.; Genina, N. QR encoded smart oral dosage forms by inkjet printing. Int. J. Pharm. 2018, 536, 138–145. [Google Scholar] [CrossRef]
- Edinger, M.; Jacobsen, J.; Bar-Shalom, D.; Rantanen, J.; Genina, N. Analytical aspects of printed oral dosage forms. Int. J. Pharm. 2018, 553, 97–108. [Google Scholar] [CrossRef]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef]
- Maroni, A.; Melocchi, A.; Parietti, F.; Foppoli, A.; Zema, L.; Gazzaniga, A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J. Control. Release 2017, 268, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Di Prima, M.; Coburn, J.; Hwang, D.; Kelly, J.; Khairuzzaman, A.; Ricles, L. Additively manufactured medical products—The FDA perspective. 3D Print. Med. 2016, 2, 2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef] [PubMed]




| Patient-Related Characteristics | Examples |
|---|---|
| Age | Organ and body functions, socioemotional status |
| Visual impairment | Blindness |
| Motoric impairment | Arm mobility, difficulty walking, manual dexterity |
| Swallowing impairment | Dysphagia |
| Cognitive impairment | Memory loss, dementia |
| Poor hand sensitivity | Control of movement and strength |
| Loss of hearing | |
| Dentition | |
| Health literacy | |
| Psychological distress | Negative perception, depressive disorders |
| Disease state | Comorbidities, disease disability |
| PK/PD | Renal and hepatic clearance |
| Psycho-social issues | Way of living, Employment status, access to caregivers |
| Product-Related Characteristics | Examples |
|---|---|
| Route of administration | Oral, inhalation, rectal, vaginal, dermal, parenteral |
| Product strength concentration | |
| Type of dosage form | Tablet, oral solution, ointment |
| Site of dermal application | Arm, feet, back |
| Appearance | Product size, shape, colour, embossing |
| Swallowability | Related to tablet size, shape, coating/waxing, liquid viscosity, mouth feel, Taste |
| Dose to therapeutic effect | Number of tablets, total volume of liquid |
| Dosing regimen | Dosing frequency, duration of treatment |
| Packaging | Inner/outer, labelling |
| Container closure system | |
| Dosing and administration devices | Syringes, applicator |
| Any handlings to be conducted prior to use | Opening capsules, measuring liquids, mixing |
| Instructions for use | Complexity |
| Caregiver assistance | Injections |
| Condition | Fixed Dose Combinations | Year of Marketing Authorization |
|---|---|---|
| Angina | Beta-blocker/HCN Channel blocker | 2015 |
| COPD | LABA/LAMA | 2013 |
| ICS/LABA | 2013 | |
| ICS/LABA/LAMA | 2017 | |
| Dyslipidaemia/Atherosclerosis | Statin/Cholesterol absorption inhibitor | 2004 |
| Statin/Niacin | 2008 | |
| Statin/Aspirin | 2004 | |
| DP1 anti-flushing/Niacin | 2008 | |
| Heart failure | Beta-blocker/ACEI | 2015 |
| Beta-blocker/HCN Channel blocker | 2015 | |
| ARB/Diuretic | 1998 | |
| ACEI/Diuretic | 1997 | |
| HIV | NRTI/NRTI | 1998 |
| PI/PI | 2001 | |
| NRTI/NRTI/NRTI | 2000 | |
| NRTI/NRTI/NNRTI | 2007 | |
| NRTI/NRTI/Integrase inhibitor/Booster of integrase inhibitor | 2013 | |
| Hypertension | ACEI/CCB | 2008 |
| ACEI/Diuretic | 1997 | |
| ACEI/Beta-blocker | 2015 | |
| ARB/CCB | 2007 | |
| ARB/Diuretic | 1998 | |
| CCB/Diuretic | 2013 | |
| ARB/CCB/Diuretic | 2009 | |
| ACEI/CCB/Diuretic | 2014 | |
| ACEI/CCB/Statin | 2015 | |
| ARB/Diuretic/CCB/Beta-blocker | - | |
| Osteoporosis | Bisphosphonates/Cholecalciferol | 2005 |
| Post myocardial infarction | Aspirin/Thienopyridines | 2010 |
| Beta-blocker/ACEI | 2015 | |
| Type II diabetes | Biguanide/Sulfonylurea | 2016 |
| Biguanide/Glitazon | 2003 | |
| Sulfonylurea/Glitazon | 2006 | |
| Biguanide/DPP-4 Inhibitor | 2007 | |
| Glitazon/DPP-4 Inhibitor | 2013 | |
| Biguanide/Glinid | 2008 | |
| Biguanide/Glifozin | 2014 | |
| DPP-4 Inhibitor/Glifozin | 2016 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menditto, E.; Orlando, V.; De Rosa, G.; Minghetti, P.; Musazzi, U.M.; Cahir, C.; Kurczewska-Michalak, M.; Kardas, P.; Costa, E.; Sousa Lobo, J.M.; et al. Patient Centric Pharmaceutical Drug Product Design—The Impact on Medication Adherence. Pharmaceutics 2020, 12, 44. https://doi.org/10.3390/pharmaceutics12010044
Menditto E, Orlando V, De Rosa G, Minghetti P, Musazzi UM, Cahir C, Kurczewska-Michalak M, Kardas P, Costa E, Sousa Lobo JM, et al. Patient Centric Pharmaceutical Drug Product Design—The Impact on Medication Adherence. Pharmaceutics. 2020; 12(1):44. https://doi.org/10.3390/pharmaceutics12010044
Chicago/Turabian StyleMenditto, Enrica, Valentina Orlando, Giuseppe De Rosa, Paola Minghetti, Umberto Maria Musazzi, Caitriona Cahir, Marta Kurczewska-Michalak, Przemysław Kardas, Elísio Costa, José Manuel Sousa Lobo, and et al. 2020. "Patient Centric Pharmaceutical Drug Product Design—The Impact on Medication Adherence" Pharmaceutics 12, no. 1: 44. https://doi.org/10.3390/pharmaceutics12010044
APA StyleMenditto, E., Orlando, V., De Rosa, G., Minghetti, P., Musazzi, U. M., Cahir, C., Kurczewska-Michalak, M., Kardas, P., Costa, E., Sousa Lobo, J. M., & Almeida, I. F. (2020). Patient Centric Pharmaceutical Drug Product Design—The Impact on Medication Adherence. Pharmaceutics, 12(1), 44. https://doi.org/10.3390/pharmaceutics12010044