Directly Compressed Tablets of Free Acid Ibuprofen with Nanocellulose Featuring Enhanced Dissolution: A Side-by-Side Comparison with Commercial Oral Dosage Forms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tablet Preparation
2.3. Differential Scanning Calorimetry (DSC)
2.4. X-ray Diffraction (XRD)
2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
2.6. In Vitro Dissolution Test in Biorelevant Media
2.7. HPLC Analysis
3. Results
3.1. Differential Scanning Calorimetry (DSC)
3.2. In Vitro Dissolution Tests in Biorelevant Media
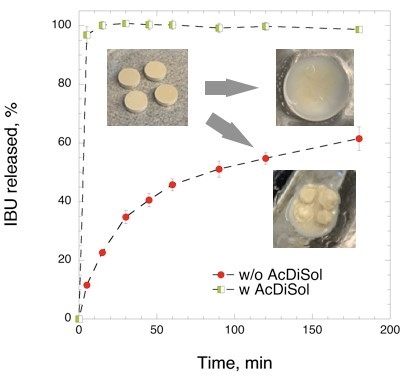
3.2.1. Effect of Superdisintegrant on IBU Release from Directly Compressed Tablets
3.2.2. Side-by-Side Comparison with Commercial Formulations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IBU | ibuprofen |
| MCC | microcrystalline cellulose |
| CLAD | Cladophora cellulose |
| DSC | differential scanning calorimetry |
| SGF | simulated gastric fluid |
| FaSIF | fasted simulated intestinal fluid |
| FeSIF | fed simulated intestinal fluid |
Appendix A
| Samples | Ton, °C | Tm, °C | ΔH, J/g drug |
|---|---|---|---|
| IBU (pure) | 75.4 ± 0.2 | 76.69 ± 0.1 | 121.41 ± 1.0 |
| 1 day | |||
| crystalline IBU | 74.89 ± 0.1 | 76.31 ± 0.2 | 6.07 ± 3.8 |
| meta-crystalline IBU | 56.07 ± 0.6 | 66.52 ± 0.1 | 60.31 ± 5.8 |
| 21 days | |||
| crystalline IBU | 75.05 ± 0.1 | 76.31 ± 0.2 | 6.07 ± 2.7 |
| meta-crystalline IBU | 56.72 ± 1.2 | 66.42 ± 0.3 | 37.91 ± 6.8 |

References
- Potthast, H.D.; Dressman, J.B.; Junginger, H.E.; Midha, K.K.; Oeser, H.; Shah, V.P.; Vogelpoel, H.; Barends, D.M. Biowaiver monographs for immediate release solid oral dosage forms: Ibuprofen. J. Pharm. Sci. 2005, 94, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, D.C.; Clementi, E. Clinical pharmacokinetics of ibuprofen arginine. Curr. Clin. Pharmacol. 2010, 5, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Saano, V.P.; Paronen, P.; Peura, P.; Vidgren, M. Relative pharmacokinetics of three oral 400 mg ibuprofen dosage forms in healthy volunteers. Int. J. Clin. Pharmacol. Ther. Toxicol. 1991, 29, 381–385. [Google Scholar] [PubMed]
- Moote, C.A. Ibuprofen arginine in the management of pain. A review. Clin. Drug Investig. 1996, 11, 1–7. [Google Scholar] [CrossRef]
- Ferrero-Cafiero, J.M.G.; Gich, I.; Puntes, M.; Martinez, J.; Ballester, M.R.; Coimbra, J.; Mathison, Y.; Tarre, M.; Font, X.; Antonijoan, R.M. Ibuprofen lysinate, quicker and less variable: Relative bioavailability compared to ibuprofen base in a pediatric suspension dosage form. Int. J. Clin. Pharm. Ther. 2015, 53, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Mantas, A.; Labbe, V.; Loryan, I.; Mihranyan, A. Amorphisation of Free Acid Ibuprofen and Other Profens in Mixtures with Nanocellulose: Dry Powder Formulation Strategy for Enhanced Solubility. Pharmaceutics 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafsson, C.; Lennholm, H.; Iversen, T.; Nystrom, C. Evaluation of surface and bulk characteristics of cellulose I powders in relation to compaction behavior and tablet properties. Drug Dev. Ind. Pharm. 2003, 29, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Mantas, A.; Mihranyan, A. Immediate-Release Nifedipine Binary Dry Powder Mixtures with Nanocellulose Featuring Enhanced Solubility and Dissolution Rate. Pharmaceutics 2019, 11, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudognon, E.; Danede, F.; Descamps, M.; Correia, N.T. Evidence for a new crystalline phase of racemic Ibuprofen. Pharm. Res. 2008, 25, 2853–2858. [Google Scholar] [CrossRef] [PubMed]
- Derollez, P.; Dudognon, E.; Affouard, F.; Danede, F.; Correia, N.T.; Descamps, M. Ab initio structure determination of phase II of racemic ibuprofen by X-ray powder diffraction. Acta Crystallogr. B 2010, 66, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Hughes, C.E.; Harris, K.D.M. New Insights into the Preparation of the Low-Melting Polymorph of Racemic Ibuprofen. Cryst. Growth Des. 2012, 12, 5839–5845. [Google Scholar] [CrossRef]






| Oral Dosage Form | Active Substance and Dose | Excipients |
|---|---|---|
| Film-coated tablet | Ibuprofen 400 mg | Anhydrous colloidal silica, magnesium stearate, croscarmellose sodium, microcrystalline cellulose (MCC), hypromellose, polydextrose (E1200) and polyethylene glycol 4000 |
| Soft gel liquid capsule | Ibuprofen 400 mg | macrogol, gelatin, sorbitol, sodium hydroxide and pure water |
| Salt conjugate tablet | Ibuprofen-d,l-Lysine 684 mg corresponding to Ibuprofen 400 mg | MCC, magnesium stearate, anhydrous colloidal silica, polyvinyl alcohol, titanium dioxide (E 171), macrogol and talc |
| Samples | Ton, °C | Tm, °C | ΔH, J/g drug/mix |
|---|---|---|---|
| IBU (pure) | 75.4 ± 0.2 | 76.7 ± 0.1 | 121.4 ± 1.0 |
| Non-heated mixture | |||
| crystalline IBU | 74.8 ± 0.1 | 76.4 ± 0.2 | 76.4 ± 12.1 |
| Heated mixture | |||
| crystalline IBU | 74.9 ± 0.1 | 76.3 ± 0.2 | 6.1 ± 3.8 |
| meta-crystalline IBU | 56.1 ± 0.6 | 66.5 ± 0.1 | 60.3 ± 5.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantas, A.; Petit, M.-A.; Mihranyan, A. Directly Compressed Tablets of Free Acid Ibuprofen with Nanocellulose Featuring Enhanced Dissolution: A Side-by-Side Comparison with Commercial Oral Dosage Forms. Pharmaceutics 2020, 12, 71. https://doi.org/10.3390/pharmaceutics12010071
Mantas A, Petit M-A, Mihranyan A. Directly Compressed Tablets of Free Acid Ibuprofen with Nanocellulose Featuring Enhanced Dissolution: A Side-by-Side Comparison with Commercial Oral Dosage Forms. Pharmaceutics. 2020; 12(1):71. https://doi.org/10.3390/pharmaceutics12010071
Chicago/Turabian StyleMantas, Athanasios, Marie-Amélie Petit, and Albert Mihranyan. 2020. "Directly Compressed Tablets of Free Acid Ibuprofen with Nanocellulose Featuring Enhanced Dissolution: A Side-by-Side Comparison with Commercial Oral Dosage Forms" Pharmaceutics 12, no. 1: 71. https://doi.org/10.3390/pharmaceutics12010071
APA StyleMantas, A., Petit, M. -A., & Mihranyan, A. (2020). Directly Compressed Tablets of Free Acid Ibuprofen with Nanocellulose Featuring Enhanced Dissolution: A Side-by-Side Comparison with Commercial Oral Dosage Forms. Pharmaceutics, 12(1), 71. https://doi.org/10.3390/pharmaceutics12010071