Recent Advances in Extracellular Vesicles as Drug Delivery Systems and Their Potential in Precision Medicine
Abstract
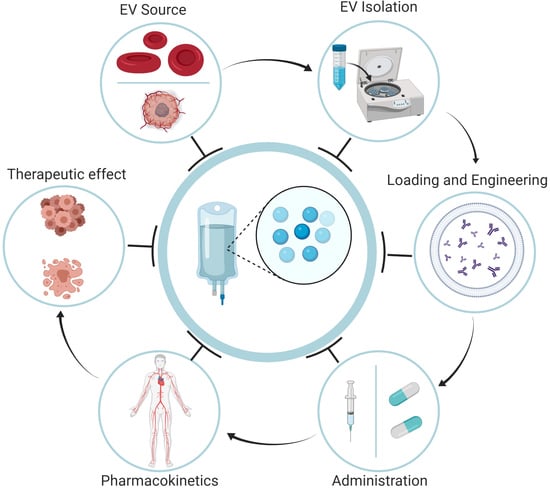
:1. Introduction
1.1. The Need for Advanced Drug Delivery Systems
1.2. Extracellular Vesicles
2. Isolation and Production of EVs
2.1. Sources of EVs
2.2. Isolation of EVs
2.3. Loading of EVs with Therapeutic Agents
2.3.1. Post-Loading Approach
2.3.2. Pre-Loading Approach
3. Administration and Biodistribution
4. EV Clearance
5. Tissue Specificity
5.1. Homing Peptides
5.2. Protein Ligands
5.3. Nucleic Acids
5.4. EV-Surface Glycosylation
6. Clinical Grade Production
7. Clinical Trials
8. Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rigalli, J.P.; Tocchetti, G.N.; Weiss, J. Modulation of ABC transporters by nuclear receptors: Physiological, pathological and pharmacological aspects. Curr. Med. Chem. 2019, 26, 1079–1112. [Google Scholar] [CrossRef]
- Arana, M.R.; Tocchetti, G.N.; Rigalli, J.P.; Mottino, A.D.; Villanueva, S.S. Physiological and pathophysiological factors affecting the expression and activity of the drug transporter MRP2 in intestine. Impact on its function as membrane barrier. Pharmacol. Res. 2016, 109, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza-Schorey, C.; Schorey, J.S. Regulation and mechanisms of extracellular vesicle biogenesis and secretion. Essays Biochem. 2018, 62, 125–133. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 23, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Kosaka, N.; Ochiya, T. Latest advances in extracellular vesicles: From bench to bedside. Sci. Technol. Adv. Mater. 2019, 20, 746–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maacha, S.; Bhat, A.A.; Jimenez, L.; Raza, A.; Haris, M.; Uddin, S.; Grivel, J.C. Extracellular vesicles-mediated intercellular communication: Roles in the tumor microenvironment and anti-cancer drug resistance. Mol. Cancer 2019, 18, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simeone, P.; Bologna, G.; Lanuti, P.; Pierdomenico, L.; Guagnano, M.T.; Pieragostino, D.; Del Boccio, P.; Vergara, D.; Marchisio, M.; Miscia, S. Extracellular vesicles as signaling mediators and disease biomarkers across biological barriers. Int. J. Mol. Sci. 2020, 21, 2514. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [Green Version]
- Wolfram, J.; Ferrari, M. Clinical cancer nanomedicine. Nano Today 2019, 25, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Badawi, M.; Pomeroy, S.; Sutaria, D.S.; Xie, Z.; Baek, A.; Jiang, J.; Elgamal, O.A.; Mo, X.; Perle, K.; et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles 2017, 6, 1324730. [Google Scholar] [CrossRef] [PubMed]
- Muntasell, A.; Berger, A.C.; Roche, P.A. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 2007, 26, 4263–4272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolte-’t Hoen, E.N.; Buschow, S.I.; Anderton, S.M.; Stoorvogel, W.; Wauben, M.H. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood 2009, 113, 1977–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Vincent-Schneider, H.; Stumptner-Cuvelette, P.; Lankar, D.; Pain, S.; Raposo, G.; Benaroch, P.; Bonnerot, C. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int. Immunol. 2002, 14, 713–722. [Google Scholar] [CrossRef]
- Théry, C.; Duban, L.; Segura, E.; Véron, P.; Lantz, O.; Amigorena, S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat. Immunol. 2002, 3, 1156–1162. [Google Scholar] [CrossRef]
- Sukhikh, G.T.; Pekarev, O.G.; Maiborodin, I.V.; Silachev, D.N.; Shevtsova, Y.A.; Goryunov, K.V.; Onoprienko, N.V.; Maiborodina, V.I.; Galenok, R.V.; Novikov, A.V.; et al. Preservation of mesenchymal stem cell-derived extracellular vesicles after abdominal delivery in the experiment. Bull. Exp. Biol. Med. 2020, 169, 122–129. [Google Scholar] [CrossRef]
- Lee, C.; Mitsialis, S.A.; Aslam, M.; Vitali, S.H.; Vergadi, E.; Konstantinou, G.; Sdrimas, K.; Fernandez-Gonzalez, A.; Kourembanas, S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012, 126, 2601–2611. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.L.; Wang, Z.G.; Liu, L.; Feng, Y.H.; Wang, J.X.; Xie, H.C.; Yang, X.L.; Li, J.F.; Feng, G.W. Immature dendritic cells derived exosomes promotes immune tolerance by regulating T cell differentiation in renal transplantation. Aging 2019, 26, 8911–8924. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Zhao, Y.; Jin, Y.S.; Li, S.M.; Bago, J.R.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Macrophage-derived extracellular vesicles as drug delivery systems for triple negative breast cancer (TNBC) therapy. J. Neuroimmune Pharmacol. 2020, 15, 487–500. [Google Scholar] [CrossRef]
- Zheng, H.C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Yang, Y.; Oh, S.J.; Hong, Y.; Seo, M.; Jang, M. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J. Control. Release 2017, 266, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Villa, A.; Crescenti, D.; Marzagalli, M.; Kuryk, L.; Limonta, P.; Mazzaferro, V.; Ciana, P. Heterologous and cross-species tropism of cancer-derived extracellular vesicles. Theranostics 2019, 9, 5681–5693. [Google Scholar] [CrossRef]
- Burgio, S.; Noori, L.; Marino Gammazza, A.; Campanella, C.; Logozzi, M.; Fais, S.; Bucchieri, F.; Cappello, F.; Caruso Bavisotto, C. Extracellular vesicles-based drug delivery systems: A new challenge and the exemplum of malignant pleural mesothelioma. Int. J. Mol. Sci. 2020, 21, 5432. [Google Scholar] [CrossRef] [PubMed]
- Rohde, E.; Pachler, K.; Gimona, M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy 2019, 21, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Bie, N.; Yong, T.; Tang, K.; Shi, X.; Wei, Z.; Jia, H.; Zhang, X.; Zhao, H.; Huang, W.; et al. The softness of tumour-cell-derived microparticles regulates their drug-delivery efficiency. Nat. Biomed. Eng. 2019, 3, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Shiri, F.; Gale, B.K.; Sant, H.; Bardi, G.T.; Hood, J.L.; Petersen, K.E. Characterization of human glioblastoma versus normal plasma-derived extracellular vesicles preisolated by differential centrifugation using cyclical electrical field-flow fractionation. Anal. Chem. 2020, 92, 9866–9876. [Google Scholar] [CrossRef] [PubMed]
- Kamyabi, N.; Abbasgholizadeh, R.; Maitra, A.; Ardekani, A.; Biswal, S.L.; Grande-Allen, K.J. Isolation and mutational assessment of pancreatic cancer extracellular vesicles using a microfluidic platform. Biomed. Microdevices 2020, 22, 23. [Google Scholar] [CrossRef]
- Lennon, K.M.; Wakefield, D.L.; Maddox, A.L.; Brehove, M.S.; Willner, A.N.; Garcia-Mansfield, K.; Meechoovet, B.; Reiman, R.; Hutchins, E.; Miller, M.M.; et al. Single molecule characterization of individual extracellular vesicles from pancreatic cancer. J. Extracell. Vesicles 2019, 8, 1685634. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Park, Y.H.; Kim, Y.G.; Lee, J.Y.; Park, J. Aqueous two-phase system to isolate extracellular vesicles from urine for prostate cancer diagnosis. PLoS ONE 2018, 13, e0194818. [Google Scholar] [CrossRef]
- Liang, L.G.; Sheng, Y.F.; Zhou, S.; Inci, F.; Li, L.; Demirci, U.; Wang, S. An integrated double-filtration microfluidic device for detection of extracellular vesicles from urine for bladder cancer diagnosis. Methods Mol. Biol. 2017, 1660, 355–364. [Google Scholar] [CrossRef]
- Kagota, S.; Taniguchi, K.; Lee, S.W.; Ito, Y.; Kuranaga, Y.; Hashiguchi, Y.; Inomata, Y.; Imai, Y.; Tanaka, R.; Tashiro, K.; et al. Analysis of extracellular vesicles in gastric juice from gastric cancer patients. Int. J. Mol. Sci. 2019, 20, 953. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, N.; Hong, C.S.; Ludwig, S.; Azambuja, J.H.; Sharma, P.; Theodoraki, M.N.; Whiteside, T.L. Isolation and analysis of tumor-derived exosomes. Curr. Protoc. Immunol. 2019, 127, e91. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.A.C.; Bussolati, B.; D’Antico, S.; Ghiotto, S.; Tetta, C.; Brizzi, M.F.; Camussi, G. Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor miRNAs. Mol. Ther. Methods Clin. Dev. 2019, 13, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez-Ríos, A.J.; Molina-Crespo, Á.; Bouzo, B.L.; López-López, R.; Moreno-Bueno, G.; de la Fuente, M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J. Nanobiotechnol. 2019, 17, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L.; et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Control Release 2018, 287, 156–166. [Google Scholar] [CrossRef]
- Yu, G.; Jung, H.; Kang, Y.Y.; Mok, H. Comparative evaluation of cell- and serum-derived exosomes to deliver immune stimulators to lymph nodes. Biomaterials 2018, 162, 71–81. [Google Scholar] [CrossRef]
- Sun, I.O.; Lerman, L.O. Urinary extracellular vesicles as biomarkers of kidney disease: From diagnostics to therapeutics. Diagnostics 2020, 10, 311. [Google Scholar] [CrossRef]
- Nakamichi, E.; Sakakura, H.; Mii, S.; Yamamoto, N.; Hibi, H.; Asai, M.; Takahashi, M. Detection of serum/salivary exosomal Alix in patients with oral squamous cell carcinoma. Oral Dis. 2020, in press. [Google Scholar] [CrossRef]
- Ling, X.; Zhang, G.; Xia, Y.; Zhu, Q.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Yang, Y.; Wang, Y.; et al. Exosomes from human urine-derived stem cells enhanced neurogenesis via miR-26a/HDAC6 axis after ischaemic stroke. J. Cell Mol. Med. 2020, 24, 640–654. [Google Scholar] [CrossRef] [Green Version]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.G.; Guo, P. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandham, S.; Su, X.; Wood, J.; Nocera, A.L.; Alli, S.C.; Milane, L.; Zimmerman, A.; Amiji, M.; Ivanov, A.R. Technologies and standardization in research on extracellular vesicles. Trends Biotechnol. 2020, 38, 1066–1098. [Google Scholar] [CrossRef] [PubMed]
- Laulagnier, K.; Vincent-Schneider, H.; Hamdi, S.; Subra, C.; Lankar, D.; Record, M. Characterization of exosome subpopulations from RBL-2H3 cells using fluorescent lipids. Blood Cells Mol. Dis. 2005, 35, 116–121. [Google Scholar] [CrossRef]
- Smith, J.A.; Leonardi, T.; Huang, B.; Iraci, N.; Vega, B.; Pluchino, S. Extracellular vesicles and their synthetic analogues in aging and age-associated brain diseases. Biogerontology 2015, 16, 147–185. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Han, C.; Labuz, J.M.; Kim, J.; Kim, J.; Cho, S.; Gho, Y.S.; Takayama, S.; Park, J. High-yield isolation of extracellular vesicles using aqueous two-phase system. Sci. Rep. 2015, 14. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Kolluri, K.K.; Gowers, K.H.; Janes, S.M. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J. Extracell. Vesicles 2017, 6, 1265291. [Google Scholar] [CrossRef]
- Yim, N.; Ryu, S.W.; Choi, K.; Lee, K.R.; Lee, S.; Choi, H.; Kim, J.; Shaker, M.R.; Sun, W.; Park, J.H.; et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat. Commun. 2016, 7, 12277. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, J.; Tong, A.; Yang, H. Oncolytic viruses for cancer therapy: Barriers and recent advances. Mol. Ther. Oncolytics 2019, 15, 234–247. [Google Scholar] [CrossRef] [Green Version]
- Gould, S.J.; Booth, A.M.; Hildreth, J.E. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592–10597. [Google Scholar] [CrossRef] [Green Version]
- Garofalo, M.; Saari, H.; Somersalo, P.; Crescenti, D.; Kuryk, L.; Aksela, L.; Capasso, C.; Madetoja, M.; Koskinen, K.; Oksanen, T.; et al. Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment. J. Control. Release 2018, 283, 223–234. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Gong, C.; Wang, Z.; Xia, Q.; Gu, F.; Hu, C.; Zhang, L.; Guo, H.; Gao, S. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine 2018, 14, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Kalinec, G.M.; Gao, L.; Cohn, W.; Whitelegge, J.P.; Faull, K.F.; Kalinec, F. Extracellular vesicles from auditory cells as nanocarriers for anti-inflammatory drugs and pro-resolving mediators. Front Cell Neurosci. 2019, 13, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinec, G.M.; Cohn, W.; Whitelegge, J.P.; Faull, K.F.; Kalinec, F. Preliminary characterization of extracellular vesicles from auditory HEI-OC1 cells. Ann. Otol. Rhinol. Laryngol. 2019, 128, S52–S60. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Jay, S.M. Production of extracellular vesicles loaded with therapeutic cargo. Methods Mol. Biol. 2018, 1831, 37–47. [Google Scholar] [CrossRef]
- Saari, H.; Lázaro-Ibáñez, E.; Viitala, T.; Vuorimaa-Laukkanen, E.; Siljander, P.; Yliperttula, M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J. Control. Release 2015, 220, 727–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. Int. J. Nanomed. 2019, 14, 8603–8610. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, J.; Wu, J.; Fan, Q.; Zhou, J.; Wu, J.; Liu, S.; Zang, J.; Ye, J.; Xiao, M.; et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J. Nanobiotechnol. 2019, 17, 29. [Google Scholar] [CrossRef] [Green Version]
- Perteghella, S.; Crivelli, B.; Catenacci, L.; Sorrenti, M.; Bruni, G.; Necchi, V.; Vigani, B.; Sorlini, M.; Torre, M.L.; Chlapanidas, T. Stem cell-extracellular vesicles as drug delivery systems: New frontiers for silk/curcumin nanoparticles. Int. J. Pharm. 2017, 520, 86–97. [Google Scholar] [CrossRef]
- O’Loughlin, A.J.; Mäger, I.; De Jong, O.G.; Varela, M.A.; Schiffelers, R.M.; El Andaloussi, S.; Wood, M.J.A.; Vader, P. Functional delivery of lipid-conjugated siRNA by extracellular vesicles. Mol. Ther. 2017, 25, 1580–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Shi, G.; Guo, J.; Wang, C.; He, Y. Exosome-encapsulated antibiotic against intracellular infections of methicillin-resistant Staphylococcus aureus. Int. J. Nanomed. 2018, 13, 8095–8104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.K.; Ménager, C.; Wilhelm, C. Magnetic drug carriers: Bright insights from light-responsive magnetic liposomes. Nanomedicine 2015, 10, 2797–2799. [Google Scholar] [CrossRef] [Green Version]
- Salarpour, S.; Forootanfar, H.; Pournamdari, M.; Ahmadi-Zeidabadi, M.; Esmaeeli, M.; Pardakhty, A. Paclitaxel incorporated exosomes derived from glioblastoma cells: Comparative study of two loading techniques. Daru 2019, 27, 533–539. [Google Scholar] [CrossRef]
- Wu, Y.W.; Huang, C.C.; Changou, C.A.; Lu, L.S.; Goubran, H.; Burnouf, T. Clinical-grade cryopreserved doxorubicin-loaded platelets: Role of cancer cells and platelet extracellular vesicles activation loop. J. Biomed. Sci. 2020, 27, 45. [Google Scholar] [CrossRef] [Green Version]
- Carobolante, G.; Mantaj, J.; Ferrari, E.; Vllasaliu, D. Cow Milk and intestinal epithelial cell-derived extracellular vesicles as systems for enhancing oral drug delivery. Pharmaceutics 2020, 12, 226. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, X.; Tian, B.; Liu, J.; Yang, L.; Zeng, L.; Chen, T.; Hong, A.; Wang, X. Nucleolin-targeted extracellular vesicles as a versatile platform for biologics delivery to breast cancer. Theranostics 2017, 7, 1360–1372. [Google Scholar] [CrossRef]
- Martins-Marques, T.; Pinho, M.J.; Zuzarte, M.; Oliveira, C.; Pereira, P.; Sluijter, J.P.; Gomes, C.; Girao, H. Presence of Cx43 in extracellular vesicles reduces the cardiotoxicity of the anti-tumour therapeutic approach with doxorubicin. J. Extracell. Vesicles 2016, 5, 32538. [Google Scholar] [CrossRef]
- Limoni, S.K.; Moghadam, M.F.; Moazzeni, S.M.; Gomari, H.; Salimi, F. Engineered exosomes for targeted transfer of siRNA to HER2 positive breast cancer cells. Appl. Biochem. Biotechnol. 2019, 187, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Faruqu, F.N.; Xu, L.; Al-Jamal, K.T. Preparation of exosomes for siRNA delivery to cancer cells. J. Vis. Exp. 2018, 142, e58814. [Google Scholar] [CrossRef]
- Schindler, C.; Collinson, A.; Matthews, C.; Pointon, A.; Jenkinson, L.; Minter, R.R.; Vaughan, T.J.; Tigue, N.J. Exosomal delivery of doxorubicin enables rapid cell entry and enhanced in vitro potency. PLoS ONE 2019, 14, e0214545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, T.N.; Raiker, R.S.; Jay, S.M. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol. Pharm. 2015, 12, 3650–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.O.; Jo, H.; Yu, J.H.; Gambhir, S.S.; Pratx, G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials 2018, 177, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Gomari, H.; Forouzandeh Moghadam, M.; Soleimani, M.; Ghavami, M.; Khodashenas, S. Targeted delivery of doxorubicin to HER2 positive tumor models. Int. J. Nanomed. 2019, 14, 5679–5690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, X.; Du, L.; Wang, Y.; Liu, X.; Tian, H.; Wang, L.; Li, P.; Zhao, Y.; Duan, W.; et al. Exosome-transmitted miR-128-3p increase chemosensitivity of oxaliplatin-resistant colorectal cancer. Mol. Cancer 2019, 18, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Jhan, Y.Y.; Prasca-Chamorro, D.; Palou Zuniga, G.; Moore, D.M.; Arun Kumar, S.; Gaharwar, A.K.; Bishop, C.J. Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery. Int. J. Pharm. 2020, 573, 118802. [Google Scholar] [CrossRef]
- Park, O.; Choi, E.S.; Yu, G.; Kim, J.Y.; Kang, Y.Y.; Jung, H.; Mok, H. Efficient delivery of tyrosinase related protein-2 (TRP2) peptides to lymph nodes using serum-derived exosomes. Macromol. Biosci. 2018, 18, e1800301. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Jeyaram, A.; Patel, D.B.; Parajuli, B.; Livingston, N.K.; Arumugasaamy, N.; Schardt, J.S.; Jay, S.M. Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell. Mol. Bioeng. 2016, 9, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Harrison, E.B.; Zhao, Y.; Kabanov, A.V.; Batrakova, E.V. TPP1 Delivery to lysosomes with extracellular vesicles and their enhanced brain distribution in the animal model of batten disease. Adv. Healthc. Mater. 2019, 8, e1801271. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.; Nguyen, T.D.T.; Marasini, R.; Aryal, S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019, 94, 482–494. [Google Scholar] [CrossRef]
- Yu, M.; Gai, C.; Li, Z.; Ding, D.; Zheng, J.; Zhang, W.; Lv, S.; Li, W. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019, 110, 3173–3182. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomedicine 2018, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Go, G.; Lee, J.; Choi, D.S.; Kim, S.S.; Gho, Y.S. Extracellular vesicle-mimetic ghost nanovesicles for delivering anti-inflammatory drugs to mitigate gram-negative bacterial outer membrane vesicle-induced systemic inflammatory response syndrome. Adv. Healthc. Mater. 2019, 8, e1801082. [Google Scholar] [CrossRef]
- Matsuda, A.; Moirangthem, A.; Angom, R.S.; Ishiguro, K.; Driscoll, J.; Yan, I.K.; Mukhopadhyay, D.; Patel, T. Safety of bovine milk derived extracellular vesicles used for delivery of RNA therapeutics in zebrafish and mice. J. Appl. Toxicol. 2020, 40, 706–718. [Google Scholar] [CrossRef]
- Hu, G.; Liao, K.; Niu, F.; Yang, L.; Dallon, B.W.; Callen, S.; Tian, C.; Shu, J.; Cui, J.; Sun, Z.; et al. Astrocyte EV-induced lincRNA-Cox2 regulates microglial phagocytosis: Implications for morphine-mediated neurodegeneration. Mol. Ther. Nucleic Acids 2018, 13, 450–463. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, H.; Bai, M.; Ning, T.; Ge, S.; Deng, T.; Liu, R.; Zhang, L.; Ying, G.; Ba, Y. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol. Ther. 2018, 26, 774–783. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.Y.; Park, H.; Kim, H.; Mun, D.; Park, H.; Yun, N.; Joung, B. Human peripheral blood-derived exosomes for microRNA delivery. Int. J. Mol. Med. 2019, 43, 2319–2328. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wang, S.; Wang, Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials 2017, 135, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Monfared, H.; Jahangard, Y.; Nikkhah, M.; Mirnajafi-Zadeh, J.; Mowla, S.J. Potential therapeutic effects of exosomes packed with a mir-21-sponge construct in a rat model of glioblastoma. Front. Oncol. 2019, 9, 782. [Google Scholar] [CrossRef] [Green Version]
- Sutaria, D.S.; Jiang, J.; Elgamal, O.A.; Pomeroy, S.M.; Badawi, M.; Zhu, X.; Pavlovicz, R.; Azevedo-Pouly, A.C.P.; Chalmers, J.; Li, C.; et al. Low active loading of cargo into engineered extracellular vesicles results in inefficient miRNA mimic delivery. J. Extracell. Vesicles 2017, 6, 1333882. [Google Scholar] [CrossRef] [PubMed]
- Heath, N.; Osteikoetxea, X.; de Oliveria, T.M.; Lázaro-Ibáñez, E.; Shatnyeva, O.; Schindler, C.; Tigue, N.; Mayr, L.M.; Dekker, N.; Overman, R.; et al. Endosomal escape enhancing compounds facilitate functional delivery of extracellular vesicle cargo. Nanomedicine 2019, 14, 2799–2814. [Google Scholar] [CrossRef]
- Bliss, S.A.; Sinha, G.; Sandiford, O.A.; Williams, L.M.; Engelberth, D.J.; Guiro, K.; Isenalumhe, L.L.; Greco, S.J.; Ayer, S.; Bryan, M.; et al. Mesenchymal stem cell-derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Res. 2016, 76, 5832–5844. [Google Scholar] [CrossRef] [Green Version]
- Bose, R.J.C.; Uday Kumar, S.; Zeng, Y.; Afjei, R.; Robinson, E.; Lau, K.; Bermudez, A.; Habte, F.; Pitteri, S.J.; Sinclair, R.; et al. Tumor cell-derived extracellular vesicle-coated nanocarriers: An efficient theranostic platform for the cancer-specific delivery of anti-miR-21 and imaging agents. ACS Nano 2018, 12, 10817–10832. [Google Scholar] [CrossRef]
- Hung, M.E.; Leonard, J.N. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J. Extracell. Vesicles 2016, 5, 31027. [Google Scholar] [CrossRef] [PubMed]
- You, H.J.; Fang, S.B.; Wu, T.T.; Zhang, H.; Feng, Y.K.; Li, X.J.; Yang, H.H.; Li, G.; Li, X.H.; Wu, C.; et al. Mesenchymal stem cell-derived exosomes improve motor function and attenuate neuropathology in a mouse model of Machado-Joseph disease. Stem Cell. Res. Ther. 2020, 11, 222. [Google Scholar] [CrossRef]
- Brossa, A.; Fonsato, V.; Grange, C.; Tritta, S.; Tapparo, M.; Calvetti, R.; Cedrino, M.; Fallo, S.; Gontero, P.; Camussi, G.; et al. Extracellular vesicles from human liver stem cells inhibit renal cancer stem cell-derived tumor growth in vitro and in vivo. Int. J. Cancer 2020, 147, 1694–1706. [Google Scholar] [CrossRef] [Green Version]
- Campanella, C.; Caruso Bavisotto, C.; Logozzi, M.; Marino Gammazza, A.; Mizzoni, D.; Cappello, F.; Fais, S. On the choice of the extracellular vesicles for therapeutic purposes. Int. J. Mol. Sci. 2019, 20, 236. [Google Scholar] [CrossRef] [Green Version]
- Ubogu, E.E. Biology of the human blood-nerve barrier in health and disease. Exp. Neurol. 2020, 328, 113272. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.; Zhao, B.; Wang, C. Exosomes derived from miR-544-modified mesenchymal stem cells promote recovery after spinal cord injury. Arch. Physiol. Biochem. 2020, 126, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, Y.; Kong, C.; Su, X.; Zhang, X.; Bai, W.; He, X. MiR-124 enriched exosomes promoted the M2 polarization of microglia and enhanced hippocampus neurogenesis after traumatic brain injury by inhibiting TLR4 pathway. Neurochem. Res. 2019, 44, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef]
- Bonafede, R.; Turano, E.; Scambi, I.; Busato, A.; Bontempi, P.; Virla, F.; Schiaffino, L.; Marzola, P.; Bonetti, B.; Mariotti, R. ASC-exosomes ameliorate the disease progression in SOD1(G93A) murine model underlining their potential therapeutic use in human ALS. Int. J. Mol. Sci. 2020, 21, 3651. [Google Scholar] [CrossRef]
- Kim, G.; Kim, M.; Lee, Y.; Byun, J.W.; Hwang, D.W.; Lee, M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J. Control. Release 2020, 317, 273–281. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Jia, L.; Lyu, J.; Sun, Y.; Yu, H.; Li, H.; Liu, W.; Weng, Y.; Yu, W. Exosomes mediate hippocampal and cortical neuronal injury induced by hepatic ischemia-reperfusion injury through activating pyroptosis in rats. Oxid. Med. Cell Longev. 2019, 2019, 3753485. [Google Scholar] [CrossRef]
- Monroe, M.N.; Zhaorigetu, S.; Gupta, V.S.; Jin, D.; Givan, K.D.; Curylo, A.L.; Olson, S.D.; Cox, C.S.; Segura, A.; Buja, L.M.; et al. Extracellular vesicles influence the pulmonary arterial extracellular matrix in congenital diaphragmatic hernia. Pediatr. Pulmonol. 2020. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, C.; Zou, X.; Shen, W.; Yang, J.; Zhang, X.; Hu, X.; Wang, H.; Liao, Y.; Jing, T. Exosomes derived from mesenchymal stem cells inhibit neointimal hyperplasia by activating the Erk1/2 signalling pathway in rats. Stem Cell. Res. Ther. 2020, 11, 220. [Google Scholar] [CrossRef]
- Gu, X.; Li, Y.; Chen, K.; Wang, X.; Wang, Z.; Lian, H.; Lin, Y.; Rong, X.; Chu, M.; Lin, J.; et al. Exosomes derived from umbilical cord mesenchymal stem cells alleviate viral myocarditis through activating AMPK/mTOR-mediated autophagy flux pathway. J. Cell Mol. Med. 2020, 24, 7515–7530. [Google Scholar] [CrossRef]
- Liu, J.; Chen, T.; Lei, P.; Tang, X.; Huang, P. Exosomes released by bone marrow mesenchymal stem cells attenuate lung injury induced by intestinal ischemia reperfusion via the TLR4/NF-κB pathway. Int. J. Med. Sci. 2019, 16, 1238–1244. [Google Scholar] [CrossRef] [Green Version]
- Mahdipour, E.; Salmasi, Z.; Sabeti, N. Potential of stem cell-derived exosomes to regenerate β islets through Pdx-1 dependent mechanism in a rat model of type 1 diabetes. J. Cell Physiol. 2019, 234, 20310–20321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lou, G.; Li, A.; Zhang, T.; Qi, J.; Ye, D.; Zheng, M.; Chen, Z. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine 2018, 36, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Liu, X.X.; Fan, H.; Tang, Q.; Shou, Z.X.; Zuo, D.M.; Zou, Z.; Xu, M.; Chen, Q.Y.; Peng, Y.; et al. extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS ONE 2015, 10, e0140551. [Google Scholar] [CrossRef] [Green Version]
- Grange, C.; Papadimitriou, E.; Dimuccio, V.; Pastorino, C.; Molina, J.; O’Kelly, R.; Niedernhofer, L.J.; Robbins, P.D.; Camussi, G.; Bussolati, B. Urinary extracellular vesicles carrying klotho improve the recovery of renal function in an acute tubular injury model. Mol. Ther. 2020, 28, 490–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Chopp, M.; Szalad, A.; Lu, X.; Zhang, Y.; Wang, X.; Cepparulo, P.; Lu, M.; Li, C.; Zhang, Z.G. Exosomes derived from schwann cells ameliorate peripheral neuropathy in type 2 diabetic mice. Diabetes 2020, 69, 749–759. [Google Scholar] [CrossRef]
- Jiang, K.; Yang, J.; Guo, S.; Zhao, G.; Wu, H.; Deng, G. peripheral circulating exosome-mediated delivery of miR-155 as a novel mechanism for acute lung inflammation. Mol. Ther. 2019, 27, 1758–1771. [Google Scholar] [CrossRef]
- Milano, G.; Biemmi, V.; Lazzarini, E.; Balbi, C.; Ciullo, A.; Bolis, S.; Ameri, P.; Di Silvestre, D.; Mauri, P.; Barile, L.; et al. Intravenous administration of cardiac progenitor cell-derived exosomes protects against doxorubicin/trastuzumab-induced cardiac toxicity. Cardiovasc. Res. 2020, 116, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Ciullo, A.; Biemmi, V.; Milano, G.; Bolis, S.; Cervio, E.; Fertig, E.T.; Gherghiceanu, M.; Moccetti, T.; Camici, G.G.; Vassalli, G.; et al. Exosomal expression of CXCR4 targets cardioprotective vesicles to myocardial infarction and improves outcome after systemic administration. Int. J. Mol. Sci. 2019, 20, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandergriff, A.; Huang, K.; Shen, D.; Hu, S.; Hensley, M.T.; Caranasos, T.G.; Qian, L.; Cheng, K. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics 2018, 8, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Guo, M.; Hu, T.; Li, W.; Huang, S.; Yin, Z.; Li, Y.; Chen, F.; Zhu, L.; Kang, C.; et al. Increased microglial exosomal miR-124-3p alleviates neurodegeneration and improves cognitive outcome after rmTBI. Mol. Ther. 2020, 28, 503–522. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Li, Z.; He, T.; Qu, M.; Jiang, L.; Li, W.; Shi, X.; Pan, J.; Zhang, L.; Wang, Y.; et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 2019, 9, 2910–2923. [Google Scholar] [CrossRef] [PubMed]
- Venkat, P.; Cui, C.; Chopp, M.; Zacharek, A.; Wang, F.; Landschoot-Ward, J.; Shen, Y.; Chen, J. MiR-126 mediates brain endothelial cell exosome treatment-induced neurorestorative effects after stroke in type 2 diabetes mellitus mice. Stroke 2019, 50, 2865–2874. [Google Scholar] [CrossRef]
- Nie, H.; Xie, X.; Zhang, D.; Zhou, Y.; Li, B.; Li, F.; Li, F.; Cheng, Y.; Mei, H.; Meng, H.; et al. Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale 2020, 12, 877–887. [Google Scholar] [CrossRef]
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 3838. [Google Scholar] [CrossRef] [Green Version]
- Maia, J.; Caja, S.; Strano Moraes, M.C.; Couto, N.; Costa-Silva, B. Exosome-based cell-cell communication in the tumor microenvironment. Front. Cell Dev. Biol. 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Kodali, M.; Castro, O.W.; Kim, D.K.; Thomas, A.; Shuai, B.; Attaluri, S.; Upadhya, R.; Gitai, D.; Madhu, L.N.; Prockop, D.J.; et al. Intranasally administered human MSC-derived extracellular vesicles pervasively incorporate into neurons and microglia in both intact and status epilepticus injured forebrain. Int. J. Mol. Sci. 2019, 21, 181. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Perets, N.; Betzer, O.; Ben-Shaul, S.; Sheinin, A.; Michaelevski, I.; Popovtzer, R.; Offen, D.; Levenberg, S. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano 2019, 13, 10015–10028. [Google Scholar] [CrossRef] [PubMed]
- Thomi, G.; Joerger-Messerli, M.; Haesler, V.; Muri, L.; Surbek, D.; Schoeberlein, A. Intranasally administered exosomes from umbilical cord stem cells have preventive neuroprotective effects and contribute to functional recovery after perinatal brain injury. Cells 2019, 8, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomi, G.; Surbek, D.; Haesler, V.; Joerger-Messerli, M.; Schoeberlein, A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res. Ther. 2019, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Scholl, J.N.; de Fraga Dias, A.; Pizzato, P.R.; Lopes, D.V.; Moritz, C.E.J.; Jandrey, E.H.F.; Souto, G.D.; Colombo, M.; Rohden, F.; Sevigny, J.; et al. Characterization and antiproliferative activity of glioma-derived extracellular vesicles. Nanomedicine 2020, 15, 1001–1018. [Google Scholar] [CrossRef] [PubMed]
- Narbute, K.; Piļipenko, V.; Pupure, J.; Dzirkale, Z.; Jonavičė, U.; Tunaitis, V.; Kriaučiūnaitė, K.; Jarmalavičiūtė, A.; Jansone, B.; Kluša, V.; et al. Intranasal administration of extracellular vesicles derived from human teeth stem cells improves motor symptoms and normalizes tyrosine hydroxylase expression in the substantia nigra and striatum of the 6-hydroxydopamine-treated rats. Stem Cells Transl. Med. 2019, 8, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.P.; Smith, V.L.; Karakousis, P.C.; Schorey, J.S. Exosomes isolated from mycobacteria-infected mice or cultured macrophages can recruit and activate immune cells in vitro and in vivo. J. Immunol. 2012, 189, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Prado, N.; Cañamero, M.; Villalba, M.; Rodríguez, R.; Batanero, E. Bystander suppression to unrelated allergen sensitization through intranasal administration of tolerogenic exosomes in mouse. Mol. Immunol. 2010, 47, 2148–2151. [Google Scholar] [CrossRef]
- Trelis, M.; Galiano, A.; Bolado, A.; Toledo, R.; Marcilla, A.; Bernal, D. Subcutaneous injection of exosomes reduces symptom severity and mortality induced by Echinostoma caproni infection in BALB/c mice. Int. J. Parasitol. 2016, 46, 799–808. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, J.O.; Ha, D.H.; Yi, Y.W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res. Ther. 2018, 9, 187. [Google Scholar] [CrossRef] [Green Version]
- Del Cacho, E.; Gallego, M.; Lillehoj, H.S.; Quilez, J.; Lillehoj, E.P.; Sánchez-Acedo, C. Induction of protective immunity against experimental Eimeria tenella infection using serum exosomes. Vet. Parasitol. 2016, 224, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, B.; Zhang, A.; Hassounah, F.; Seow, Y.; Wood, M.; Ma, F.; Klein, J.D.; Price, S.R.; Wang, X.H. Exosome-mediated miR-29 transfer reduces muscle atrophy and kidney fibrosis in mice. Mol. Ther. 2019, 27, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Ju, C.; Li, Y.; Shen, Y.; Liu, Y.; Cai, J.; Liu, N.; Ma, G.; Tang, Y. Transplantation of Cardiac Mesenchymal Stem Cell-Derived Exosomes for Angiogenesis. J. Cardiovasc. Transl. Res. 2018, 11, 429–437. [Google Scholar] [CrossRef]
- Hu, G.-W.; Li, Q.; Niu, X.; Hu, B.; Liu, J.; Zhou, S.-M.; Guo, S.-C.; Lang, H.-L.; Zhang, C.-Q.; Wang, Y.; et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res. Ther. 2015, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Li, Z.; Sun, W.; Yang, G.; Xing, C.; Yuan, L. Delivery efficacy differences of intravenous and intraperitoneal injection of exosomes: Perspectives from tracking dye labeled and MiRNA encapsulated exosomes. Curr. Drug Deliv. 2020, 17, 186–194. [Google Scholar] [CrossRef]
- Betker, J.L.; Angle, B.M.; Graner, M.W.; Anchordoquy, T.J. The potential of exosomes from cow milk for oral delivery. J. Pharm. Sci. 2019, 108, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Manca, S.; Upadhyaya, B.; Mutai, E.; Desaulniers, A.T.; Cederberg, R.A.; White, B.R.; Zempleni, J. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci. Rep. 2018, 8, 11321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safwat, A.; Sabry, D.; Ragiae, A.; Amer, E.; Mahmoud, R.H.; Shamardan, R.M. Adipose mesenchymal stem cells–derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J. Circ. Biomark. 2018, 7, 1849454418807827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.; Wang, L.; Li, Q.; Xu, J.; Xu, J.; Xiong, Y.; Chen, G.; Qian, H.; Jin, C.; Yu, Y.; et al. Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Res. Ther. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Shiue, S.-J.; Rau, R.-H.; Shiue, H.-S.; Hung, Y.-W.; Li, Z.-X.; Yang, K.D.; Cheng, J.-K. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury–induced pain in rats. Pain 2019, 160, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Benito-Martin, A.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Dasgupta, D.; Nakao, Y.; Mauer, A.S.; Thompson, J.M.; Sehrawat, T.S.; Liao, C.-Y.; Krishnan, A.; Lucien, F.; Guo, Q.; Liu, M.; et al. IRE1A stimulates hepatocyte-derived extracellular vesicles that promote inflammation in mice with steatohepatitis. Gastroenterology 2020, 20, 34831–34839. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xiong, N.; Guo, X.; Huang, J.; Ma, K.; Liu, L.; Xia, Y.; Shen, Y.; Li, J.; Jiang, H.; et al. Exosomes from patients with Parkinson’s disease are pathological in mice. J. Mol. Med. 2019, 97, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Jin, J.; Huang, C.; Li, J.; Luo, H.; Li, L.; Huang, Y.; Jiang, Y. Exosomes derived from septic mouse serum modulate immune responses via exosome-associated cytokines. Front. Immunol. 2019, 10, 1560. [Google Scholar] [CrossRef] [Green Version]
- Pan, T.; Jia, P.; Chen, N.; Fang, Y.; Liang, Y.; Guo, M.; Ding, X. Delayed remote ischemic preconditioning confersrenoprotection against septic acute kidney injury via exosomal miR-21. Theranostics 2019, 9, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Melzer, C.; Rehn, V.; Yang, Y.; Bähre, H.; Von Der Ohe, J.; Hass, R. Taxol-loaded MSC-derived exosomes provide a therapeutic vehicle to target metastatic breast cancer and other carcinoma cells. Cancers 2019, 11, 798. [Google Scholar] [CrossRef] [Green Version]
- Tamura, R.; Uemoto, S.; Tabata, Y. Immunosuppressive effect of mesenchymal stem cell-derived exosomes on a concanavalin A-induced liver injury model. Inflamm. Regen. 2016, 36, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xue, H.; Li, T.; Chu, X.; Xin, D.; Xiong, Y.; Qiu, W.; Gao, X.; Qian, M.; Xu, J.; et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE−/- mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem. Biophys. Res. Commun. 2019, 510, 565–572. [Google Scholar] [CrossRef]
- Zhu, L.-P.; Tian, T.; Wang, J.-Y.; He, J.-N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.-X.; Qiu, X.-T.; Li, C.-C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef]
- Wen, S.; Dooner, M.; Cheng, Y.; Papa, E.; Del Tatto, M.; Pereira, M.; Deng, Y.; Goldberg, L.; Aliotta, J.; Chatterjee, D.; et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 2016, 30, 2221–2231. [Google Scholar] [CrossRef]
- Nakano, M.; Nagaishi, K.; Konari, N.; Saito, Y.; Chikenji, T.; Mizuec, Y.; Fujimiya, M. Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci. Rep. 2016, 6, 24805. [Google Scholar] [CrossRef] [Green Version]
- Lou, G.; Chen, L.; Xia, C.; Wang, W.; Qi, J.; Li, A.; Zhao, L.; Chen, Z.; Zheng, M.; Liu, Y. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J. Exp. Clin. Cancer Res. 2020, 39, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-W.; Li, F.-X.-Z.; Liu, Y.-W.; Rao, S.-S.; Yin, H.; Huang, J.; Chen, C.-Y.; Hu, Y.; Zhang, Y.; Tan, Y.-J.; et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale 2019, 11, 20884–20892. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Yuan, M.; Zhang, T.; Wei, H.; Xu, S.; Jiang, N.; Zheng, N.; Wu, Z. Extracellular vesicles expressing a single-chain variable fragment of an HIV-1 specific antibody selectively target Env+ tissues. Theranostics 2019, 9, 5657–5671. [Google Scholar] [CrossRef]
- Zhang, K.; Shao, C.-X.; Zhu, J.-D.; Lv, X.-L.; Tu, C.-Y.; Jiang, C.; Shang, M.-J. Exosomes function as nanoparticles to transfer miR-199a-3p to reverse chemoresistance to cisplatin in hepatocellular carcinoma. Biosci. Rep. 2020, 40, 40. [Google Scholar] [CrossRef]
- Ohno, S.-I.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.; Jie, Y.; Haibo, L.; Chaoneng, W.; Dong, H.; Jianbing, Z.; Junjie, G.; Leilei, M.; Hongtao, S.; Zou, Y.; et al. Dendritic cells derived exosomes migration to spleen and induction of inflammation are regulated by CCR7. Sci. Rep. 2017, 7, srep42996. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef]
- Viñas, J.L.; Spence, M.; Gutsol, A.; Knoll, W.; Burger, D.; Zimpelmann, J.; Allan, D.S.; Burns, K.D. Receptor-ligand interaction mediates targeting of endothelial colony forming cell-derived exosomes to the kidney after ischemic injury. Sci. Rep. 2018, 8, 16320. [Google Scholar] [CrossRef]
- Yang, S.; Xia, Y.-P.; Luo, X.-Y.; Chen, S.-L.; Li, B.-W.; Ye, Z.-M.; Chen, S.-C.; Mao, L.; Jin, H.-J.; Li, Y.-N.; et al. Exosomal CagA derived from Helicobacter pylori-infected gastric epithelial cells induces macrophage foam cell formation and promotes atherosclerosis. J. Mol. Cell. Cardiol. 2019, 135, 40–51. [Google Scholar] [CrossRef]
- Sun, X.; Jung, J.-H.; Arvola, O.; Santoso, M.R.; Giffard, R.G.; Yang, P.C.; Stary, C.M. Stem cell-derived exosomes protect astrocyte cultures from in vitro ischemia and decrease injury as post-stroke intravenous therapy. Front. Cell. Neurosci. 2019, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Nishida-Aoki, N.; Tominaga, N.; Kosaka, N.; Ochiya, T. Altered biodistribution of deglycosylated extracellular vesicles through enhanced cellular uptake. J. Extracell. Vesicles 2020, 9, 1713527. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, T.; Chen, T.; Sun, J.; Luo, J.; He, J.; Wei, L.; Zeng, B.; Zhang, H.; Li, W.; et al. Hepatic exosome-derived miR-130a-3p attenuates glucose intolerance via suppressing PHLPP2 gene in adipocyte. Metabolism 2020, 103, 154006. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Li, Y.; Zhu, L.; Zhou, Z. Astrocyte-derived exosomes suppress autophagy and ameliorate neuronal damage in experimental ischemic stroke. Exp. Cell Res. 2019, 382, 111474. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhang, X.; Li, W.; Feng, Q.; Feng, H.; Tong, Y.; Rong, H.; Wang, W.; Zhang, D.; Zhang, Z.; et al. Exosomal miRNA-107 induces myeloid-derived suppressor cell expansion in gastric cancer. Cancer Manag. Res. 2019, 11, 4023–4040. [Google Scholar] [CrossRef] [PubMed]
- Erb, U.; Zhao, K.; Wang, Z.; Xiao, L.; Zöller, M. Murine and human pancreatic tumor exosome recovery in mouse serum: Diagnostic and prognostic potential and target cell delivery. Cancer Lett. 2017, 403, 1–12. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhang, T.; He, W.; Jin, H.; Liu, C.; Yang, Z.; Ren, J. Methotrexate-loaded extracellular vesicles functionalized with therapeutic and targeted peptides for the treatment of glioblastoma multiforme. ACS Appl. Mater. Interfaces 2018, 10, 12341–12350. [Google Scholar] [CrossRef]
- Yang, H.-C.; Zhang, M.; Wu, R.; Zheng, H.-Q.; Zhang, L.-Y.; Luo, J.; Li, L.-L.; Hu, X.-Q. C-C chemokine receptor type 2-overexpressing exosomes alleviated experimental post-stroke cognitive impairment by enhancing microglia/macrophage M2 polarization. World J. Stem Cells 2020, 12, 152–167. [Google Scholar] [CrossRef]
- Romanelli, P.; Bieler, L.; Scharler, C.; Pachler, K.; Kreutzer, C.; Zaunmair, P.; Jakubecova, D.; Mrowetz, H.; Benedetti, B.; Rivera, F.J.; et al. Extracellular vesicles can deliver anti-inflammatory and anti-scarring activities of mesenchymal stromal cells after spinal cord injury. Front. Neurol. 2019, 10, 1225. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Pei, S.; Han, L.; Guo, B.; Li, Y.; Duan, R.; Yao, Y.; Xue, B.; Chen, X.; Jia, Y. Mesenchymal stem cell-derived exosomes reduce a1 astrocytes via downregulation of phosphorylated NFκB P65 subunit in spinal cord injury. Cell. Physiol. Biochem. 2018, 50, 1535–1559. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Pang, Y.; Zhang, C.; Liu, L.; Bi, Y. Exosomes derived from human umbilical cord mesenchymal stem cells inhibit vein graft intimal hyperplasia and accelerate reendothelialization by enhancing endothelial function. Stem Cell Res. Ther. 2020, 11, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Zhang, F.; Lian, X.F.; Peng, W.Q.; Yin, C.Y. Mesenchymal stem cell-derived exosomes improve diabetes mellitus-induced myocardial injury and fibrosis via inhibition of TGF-β1/Smad2 signaling pathway. Cell. Mol. Biol. 2019, 65, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhao, C.; Hou, S.; Zhou, W.; Wang, B.; Chen, Y. Exosomes secreted from miRNA-29b-modified mesenchymal stem cells repaired spinal cord injury in rats. Braz. J. Med Biol. Res. 2019, 52, e8735. [Google Scholar] [CrossRef] [Green Version]
- Zuo, R.; Liu, M.; Wang, Y.; Li, J.; Wang, W.; Wu, J.; Sun, C.; Li, B.; Wang, Z.; Lan, W.; et al. BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/β-catenin signaling. Stem Cell Res. Ther. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Mi, Y.; Wu, S.; You, X.; Huang, Y.; Zhu, J.; Zhu, L. Exosomes from adipose-derived stem cells protect against high glucose-induced erectile dysfunction by delivery of corin in a streptozotocin-induced diabetic rat model. Regen. Ther. 2020, 14, 227–233. [Google Scholar] [CrossRef]
- Geng, W.; Tang, H.; Luo, S.; Lv, Y.; Liang, D.; Kang, X.; Hong, W. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am. J. Transl. Res. 2019, 11, 780–792. [Google Scholar]
- Patel, N.A.; Moss, L.D.; Lee, J.-Y.; Tajiri, N.; Acosta, S.; Hudson, C.; Parag, S.; Cooper, D.R.; Borlongan, C.V.; Bickford, P.C. Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J. Neuroinflamm. 2018, 15, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-L.; Sung, P.-H.; Chen, K.-H.; Shao, P.-L.; Yang, C.-C.; Cheng, B.-C.; Lin, K.-C.; Chen, C.-H.; Chai, H.-T.; Chang, H.-W.; et al. Adipose-derived mesenchymal stem cell-derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. Am. J. Transl. Res. 2018, 10, 1053–1070. [Google Scholar]
- Chen, K.-H.; Chen, C.-H.; Wallace, C.G.; Yuen, C.-M.; Kao, G.-S.; Chen, Y.-L.; Shao, P.-L.; Chen, Y.-L.; Chai, H.-T.; Lin, K.-C.; et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 2016, 7, 74537–74556. [Google Scholar] [CrossRef] [Green Version]
- Takov, K.; He, Z.; Johnston, H.E.; Timms, J.F.; Guillot, P.V.; Yellon, D.M.; Davidson, S.M. Small extracellular vesicles secreted from human amniotic fluid mesenchymal stromal cells possess cardioprotective and promigratory potential. Basic Res. Cardiol. 2020, 115, 1–22. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Niu, X.; Hu, B.; Chen, S.; Song, W.; Ding, J.; Zhang, C.; Wang, Y. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int. J. Biol. Sci. 2017, 13, 232–244. [Google Scholar] [CrossRef]
- Jiang, Z.-Z.; Liu, Y.-M.; Niu, X.; Yin, J.-Y.; Hu, B.; Guo, S.; Fan, Y.; Wang, Y.; Wang, N. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther. 2016, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.-S.; Tong, Y.; Qiu, Y.; Ye, C.; Wu, N.; Xiong, X.-Q.; Wang, J.-J.; Han, Y.; Zhou, Y.-B.; Zhang, F.; et al. MiR155-5p in adventitial fibroblasts-derived extracellular vesicles inhibits vascular smooth muscle cell proliferation via suppressing angiotensin-converting enzyme expression. J. Extracell. Vesicles 2019, 9, 1698795. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kim, G.; Hwang, D.W.; Lee, M. Delivery of high mobility group box-1 sirna using brain-targeting exosomes for ischemic stroke therapy. J. Biomed. Nanotechnol. 2019, 15, 2401–2412. [Google Scholar] [CrossRef]
- Dominguez, J.M.; Dominguez, J.H.; Xie, D.; Kelly, K.J. Human extracellular microvesicles from renal tubules reverse kidney ischemia-reperfusion injury in rats. PLoS ONE 2018, 13, e0202550. [Google Scholar] [CrossRef]
- Dominguez, J.H.; Liu, Y.; Gao, H.; Dominguez, J.M.; Xie, D.; Kelly, K.J. Renal tubular cell-derived extracellular vesicles accelerate the recovery of established renal ischemia reperfusion injury. J. Am. Soc. Nephrol. 2017, 28, 3533–3544. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Medalla, M.; Chang, W.; Calderazzo, S.M.; Go, V.; Tsolias, A.; Goodliffe, J.W.; Pathak, D.; De Alba, D.; Pessina, M.; Rosene, D.L.; et al. Treatment with mesenchymal-derived extracellular vesicles reduces injury-related pathology in pyramidal neurons of monkey perilesional ventral premotor cortex. J. Neurosci. 2020, 40, 3385–3407. [Google Scholar] [CrossRef]
- Ophelders, D.R.M.G.; Wolfs, T.G.A.M.; Jellema, R.K.; Zwanenburg, A.; Andriessen, P.; Delhaas, T.; Ludwig, A.-K.; Radtke, S.; Peter, A.; Janssen, L.; et al. Mesenchymal stromal cell-derived extracellular vesicles protect the fetal brain after hypoxia-ischemia. Stem Cells Transl. Med. 2016, 5, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Losurdo, M.; Pedrazzoli, M.; D’Agostino, C.; Elia, C.A.; Massenzio, F.; Lonati, E.; Mauri, M.; Rizzi, L.; Molteni, L.; Bresciani, E.; et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Transl. Med. 2020, 9, 1068–1084. [Google Scholar] [CrossRef] [PubMed]
- Perets, N.; Hertz, S.; London, M.; Offen, D. Intranasal administration of exosomes derived from mesenchymal stem cells ameliorates autistic-like behaviors of BTBR mice. Mol. Autism 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Long, Q.; Upadhya, D.; Hattiangady, B.; Kim, D.-K.; An, S.Y.; Shuai, B.; Prockop, D.J.; Shetty, A.K. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. USA 2017, 114, E3536–E3545. [Google Scholar] [CrossRef] [Green Version]
- Kalani, A.; Chaturvedi, P.; Kamat, P.K.; Maldonado, C.; Bauer, P.; Joshua, I.G.; Tyagi, S.C.; Tyagi, N. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int. J. Biochem. Cell Biol. 2016, 79, 360–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.L.; Lau, S.N.; Leaw, B.; Nguyen, H.P.T.; Salamonsen, L.A.; Saad, M.I.; Chan, S.T.; Zhu, D.; Krause, M.; Kim, C.; et al. Amnion epithelial cell-derived exosomes restrict lung injury and enhance endogenous lung repair. Stem Cells Transl. Med. 2018, 7, 180–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatnagar, S.; Shinagawa, K.; Castellino, F.J.; Schorey, J.S. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 2007, 110, 3234–3244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, N.; Marazuela, E.G.; Segura, E.; Fernández-García, H.; Villalba, M.; Théry, C.; Rodríguez, R.; Batanero, E. Exosomes from bronchoalveolar fluid of tolerized mice prevent allergic reaction. J. Immunol. 2008, 181, 1519–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezquer, F.; Quintanilla, M.E.; Morales, P.; Santapau, D.; Ezquer, M.; Kogan, M.J.; Salas-Huenuleo, E.; Herrera-Marschitz, M.; Israel, Y. Intranasal delivery of mesenchymal stem cell-derived exosomes reduces oxidative stress and markedly inhibits ethanol consumption and post-deprivation relapse drinking. Addict. Biol. 2018, 24, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Takahashi, Y.; Nishikawa, M.; Sano, K.; Kato, K.; Yamashita, T.; Imai, T.; Saji, H.; Takakura, Y. Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin-lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice. J. Pharm. Sci. 2015, 104, 705–713. [Google Scholar] [CrossRef]
- Wan, Z.; Zhao, L.; Lu, F.; Gao, X.; Dong, Y.; Zhao, Y.; Wei, M.; Yang, G.; Xing, C.; Liu, L. Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics 2020, 10, 218–230. [Google Scholar] [CrossRef]
- Qiu, X.; Li, Z.; Han, X.; Zhen, L.; Luo, C.; Liu, M.; Yu, K.; Ren, Y. Tumor-derived nanovesicles promote lung distribution of the therapeutic nanovector through repression of Kupffer cell-mediated phagocytosis. Theranostics 2019, 9, 2618–2636. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nat. Cell Biol. 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Emam, S.E.; Abu Lila, A.S.; Elsadek, N.E.; Ando, H.; Shimizu, T.; Okuhira, K.; Ishima, Y.; Mahdy, M.A.; Ghazy, F.-E.S.; Ishida, T.; et al. Cancer cell-type tropism is one of crucial determinants for the efficient systemic delivery of cancer cell-derived exosomes to tumor tissues. Eur. J. Pharm. Biopharm. 2019, 145, 27–34. [Google Scholar] [CrossRef]
- Kooijmans, S.; Fliervoet, L.; Van Der Meel, R.; Fens, M.; Heijnen, H.; Henegouwen, P.V.B.E.; Vader, P.; Schiffelers, R. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Control. Release 2016, 224, 77–85. [Google Scholar] [CrossRef]
- Antes, T.J.; Middleton, R.C.; Luther, K.M.; Ijichi, T.; Peck, K.A.; Liu, W.J.; Valle, J.; Echavez, A.K.; Marbán, E. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J. Nanobiotechnol. 2018, 16, 1–15. [Google Scholar] [CrossRef]
- Zhuang, M.; Du, D.; Pu, L.; Song, H.; Deng, M.; Long, Q.; Yin, X.; Wang, Y.; Rao, L. SPION-decorated exosome delivered BAY55-9837 targeting the pancreas through magnetism to improve the blood GLC response. Small 2019, 15, e1903135. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdee, K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood–brain barrier penetration. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Liu, Z.; Zhou, Y.; Chu, D.; Li, X.; Jiang, X.; Hou, D.; Chen, X.; Chen, Y.; et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci. Rep. 2015, 5, 17543. [Google Scholar] [CrossRef]
- Cui, G.-H.; Guo, H.-D.; Li, H.; Zhai, Y.; Gong, Z.-B.; Wu, J.; Liu, J.-S.; Dong, Y.-R.; Hou, S.-X. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun. Ageing 2019, 16, 10. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Yun, N.; Mun, D.; Kang, J.-Y.; Lee, S.-H.; Park, H.; Park, H.; Joung, B. Cardiac-specific delivery by cardiac tissue-targeting peptide-expressing exosomes. Biochem. Biophys. Res. Commun. 2018, 499, 803–808. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Ding, F.; Yang, J.; Li, J.; Gao, X.; Zhang, C.; Feng, J. Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale 2020, 12, 10854–10862. [Google Scholar] [CrossRef]
- Purushothaman, A.; Bandari, S.K.; Liu, J.; Mobley, J.A.; Brown, E.E.; Sanderson, R.D. Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions. J. Biol. Chem. 2015, 291, 1652–1663. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-H.; Forterre, A.V.; Zhao, J.; Frimannsson, D.O.; Delcayre, A.; Antes, T.J.; Efron, B.; Jeffrey, S.S.; Pegram, M.D.; Matin, A. Anti-HER2 scFv-directed extracellular vesicle-mediated mRNA-based gene delivery inhibits growth of HER2-positive human breast tumor xenografts by prodrug activation. Mol. Cancer Ther. 2018, 17, 1133–1142. [Google Scholar] [CrossRef] [Green Version]
- Royo, F.; Cossío, U.; De Angulo, A.R.; Llop, J.; Falcón-Pérez, J.M. Modification of the glycosylation of extracellular vesicles alters their biodistribution in mice. Nanoscale 2019, 11, 1531–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, R.; Uemoto, S.; Tabata, Y. Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 2017, 57, 274–284. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Yao, S.; Zhou, Z.; Shi, J.; Huang, Z.; Wu, Z. Hyaluronan decoration of milk exosomes directs tumor-specific delivery of doxorubicin. Carbohydr. Res. 2020, 493, 108032. [Google Scholar] [CrossRef]
- Choi, E.S.; Song, J.; Kang, Y.Y.; Mok, H. Mannose-modified serum exosomes for the elevated uptake to murine dendritic cells and lymphatic accumulation. Macromol. Biosci. 2019, 19, e1900042. [Google Scholar] [CrossRef]
- Wiest, E.F.; Zubair, A.C. Challenges of manufacturing mesenchymal stromal cell–derived extracellular vesicles in regenerative medicine. Cytotherapy 2020, S1465–S3249. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef] [PubMed]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.-C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, 99263. [Google Scholar] [CrossRef] [PubMed]
- Pachler, K.; Lener, T.; Streif, D.; Dunai, Z.A.; Desgeorges, A.; Feichtner, M.; Öller, M.; Schallmoser, K.; Rohde, E.; Gimona, M. A Good Manufacturing Practice–grade standard protocol for exclusively human mesenchymal stromal cell–derived extracellular vesicles. Cytotherapy 2017, 19, 458–472. [Google Scholar] [CrossRef] [Green Version]
- Watson, D.C.; Yung, B.C.; Bergamaschi, C.; Chowdhury, B.; Bear, J.; Stellas, D.; Morales-Kastresana, A.; Jones, J.C.; Felber, B.K.; Chen, X.; et al. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J. Extracell. Vesicles 2018, 7, 1442088. [Google Scholar] [CrossRef] [PubMed]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.-L.; Dickson, D.W.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells 2018, 7, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bari, E.; Perteghella, S.; Catenacci, L.; Sorlini, M.; Croce, S.; Mantelli, M.; A Avanzini, M.; Sorrenti, M.; Torre, M.L. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine 2019, 14, 753–765. [Google Scholar] [CrossRef]
- Bari, E.; Perteghella, S.; Di Silvestre, D.; Sorlini, M.; Catenacci, L.; Tripodo, G.; Marrubini, G.; Rossi, R.; Tripodo, G.; Mauri, G.; et al. Pilot production of mesenchymal stem/stromal freeze-dried secretome for cell-free regenerative nanomedicine: A validated GMP-compliant process. Cells 2018, 7, 190. [Google Scholar] [CrossRef] [Green Version]
- Andriolo, G.; Provasi, E.; Cicero, V.L.; Brambilla, A.; Soncin, S.; Torre, T.; Milano, G.; Biemmi, V.; Vassalli, G.; Turchetto, L.; et al. Exosomes from human cardiac progenitor cells for therapeutic applications: Development of a GMP-grade manufacturing method. Front. Physiol. 2018, 9, 1169. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Ha, D.H.; Go, H.-K.; Youn, J.; Kim, H.-K.; Jin, R.C.; Miller, R.B.; Kim, D.-H.; Cho, B.S.; Yi, Y.W. Reproducible large-scale isolation of exosomes from adipose tissue-derived mesenchymal stem/stromal cells and their application in acute kidney injury. Int. J. Mol. Sci. 2020, 21, 4774. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov (accessed on 30 July 2020).




| Loading Method | EV Source | Therapeutic Agent or Experimental Cargo | Loading Efficiency | Model | Ref. |
|---|---|---|---|---|---|
| Passive incubation | Cell culture (LIM1215 cells) | Doxorubicin | Spectrofluorometry | Cell culture, mice tumor xenograft | [52] |
| Cell culture (HEI-OC1 cells) | Aspirin, arachidonic acid, eicosapentaenoic acid, docosohexaenoic acid, linoleic acid, lipoxin, resolvin D1 | [53] | |||
| Cell culture (HEI-OC1 cells) | Dexamethasone | [54] | |||
| Cell culture (HEK293T cells) | siRNA and rhodamine | Spectrofluorometry | [55] | ||
| Cell culture (LNCaP and PC-3 cells) | Paclitaxel | UPLC | Cell culture | [56] | |
| Cell culture (LL/2, MC-38, A549 cells), liver tissue | Oncolytic adenovirus | Cell culture and mice with colon adenocarcinoma xenograft | [23] | ||
| Cell culture (MDA-MB-231, MSCs, hESCs, HUVECs) | Different porphyrins | Cell culture | [57] | ||
| Cell culture (MSCs) | Doxorubicin | Spectrophotometry | Cell culture | [58] | |
| Cell culture (MSC) | miR-210 | Mice | [59] | ||
| Cell culture (MSC) | Nanoparticles loaded with curcumin | Spectrophotometry | [60] | ||
| Cell culture (Neuro2A, dendritic cells) | siRNA | Spectrofluorometry | Cell culture | [61] | |
| Cell culture (Raw 264.7 cells) | Linezolid | HPLC | Mice with MRSA infection | [62] | |
| Cell culture (Raw 264.7 cells) | Paclitaxel, doxorubicin | HPLC | Cell culture, different xenograft mouse models | [20] | |
| Cell culture (Raw 264.7 cells) | Paclitaxel, doxorubicin | HPLC | MDCK-MDR1 cells; mice | [63] | |
| Cell culture (Raw 264.7 cells) | Catalase | Catalase enzymatic activity | Cell culture, mice | [64] | |
| Cell culture (THP-1) | Doxorubicin, t-PA, photosensitizer molecules | Spectrofluorometry | Cell culture | [65] | |
| Cell culture (U87 cells) | Paclitaxel | HPLC | Cell culture | [66] | |
| Platelets | Doxorubicin | Spectrofluorometry | Cell culture | [67] | |
| Bovine milk | Withaferin, anthocyanidins, curcumin, paclitaxel, docetaxel | Spectrophotometry, UPLC | Rats, mice with different xenograft variants | [41] | |
| Bovine milk and cell culture | Curcumin | Cell culture | [68] | ||
| Blood | Dopamine | LC-MS | Mice | [36] | |
| Electroporation | Cell culture (Dendritic cells) | Let-7-miRNA and siRNA | qPCR | Mice with breast tumor xenograft | [69] |
| Cell culture (HEI-OC1 cells) | Aspirin, arachidonic acid, eicosapentaenoic acid, docosohexaenoic acid, linoleic acid, lipoxin, resolvin D1 | [53] | |||
| Cell culture (HEK293T cells) | Doxorubicin | Spectrofluorometry | Cell culture, mice with breast tumor xenograft | [70] | |
| Cell culture (HEK293T cells) | siRNA, rhodamine | Spectrofluorometry | [55] | ||
| Cell culture (HEK293T cells) | siRNA | Spectrofluorometry | Cell culture | [71] | |
| Cell culture (HEK293T cells) | siRNA | Cell culture | [72] | ||
| Cell culture (HEK293T cells) | Doxorubicin | Spectrofluorometry | Cell culture | [73] | |
| Cell culture (HEK293T cells, HUVECs) | dsDNA | Spectrophotometry | Cell culture | [74] | |
| Cell culture (MDA-MB-231, MSCs, hESCs, HUVECs) | Different porphyrins | Cell culture | [57] | ||
| Cell culture (MDA-MB-231 cells) | Superparamagnetic iron nanoparticles and olaparib | Cell culture, mice with breast tumor xenograft | [75] | ||
| Cell culture (MSCs) | Doxorubicin | Spectrofluorometry | Mice with multiple xenograft variants | [76] | |
| Cell culture (Normal intestinal fetal human cells) | miRNA-128-3p | qPCR | Cell culture, mice with colon tumor xenograft | [77] | |
| Cell culture (Raw 264.7 cells) | Superparamagnetic iron nanoparticles loaded with curcumin | Spectrophotometry | Mice bearing glioma cells | [78] | |
| Cell culture (Raw 264.7 cells) | Paclitaxel, doxorubicin | HPLC | MDCK-MDR1 cells; mice | [63] | |
| Cell culture (3T3 and A549 cells) | siRNA | Cell culture | [79] | ||
| Plasma and cell culture | Curcumin, siRNA, DNA-CH, miR-145 mimics | Mice with lung tumor xenograft | [35] | ||
| Plasma | miR-31-5p, miR-451a | Cell culture, | [34] | ||
| Serum | Tyrosinase-related-protein-2 | Spectrofluorometry | Cell culture | [80] | |
| Sonication | Cell culture (HEI-OC1 cells) | Dexamethasone | [54] | ||
| Cell culture (HEK293T, MCF7 cells) | siRNA, miRNA, ssDNA | Cell culture | [81] | ||
| Cell culture (IC21 cells) | Tripeptidyl peptidase-1 | Enzymatic activity | Cell culture, mice | [82] | |
| Cell culture (J774A.1 cells) | Doxorubicin | Cell culture | [83] | ||
| Cell culture (Human fetal lung fibroblast 1) | Erastin | HPLC | Cell culture | [84] | |
| Cell culture (Raw 264.7 cells) | Catalase | Catalase enzymatic activity | Cell culture, mice | [64] | |
| Cell culture (Raw 264.7 cells) | Paclitaxel | HPLC | Cell culture, mice | [85] | |
| Cell culture (Raw 264.7 cells) | Paclitaxel, doxorubicin | HPLC | MDCK-MDR1 cells; mice | [63] | |
| Cell culture (Raw 264.7 cells) | Paclitaxel, doxorubicin | HPLC | Cell culture, mice with breast cancer xenograft | [20] | |
| Cell culture (U937 cells) | Dexamethasone | HPLC | Cell culture, mice | [86] | |
| Cell culture (U87 cells) | Paclitaxel | HPLC | Cell culture | [66] | |
| Lipofection | Bovine milk | siRNA | Zebrafish and mice | [87] | |
| Transfection | Cell culture (A172 cells) | siRNA | RT-PCR | Mice | [88] |
| Cell culture (HEK293T cells) | Anti-miR-214 | Cell culture, mice | [89] | ||
| Human peripheral blood cells | miR-21 | Cell culture, mice | [90] | ||
| Saponin permeabilization | Cell culture (IC21 cells) | Tripeptidyl-peptidase-1 | Enzymatic activity | Cell culture, mice | [82] |
| Cell culture (MDA-MB-231, MSCs, hESCs, HUVECs) | Different porphyrins | Cell culture | [57] | ||
| Cell culture (Raw 264.7 cells) | Catalase | Catalase enzymatic activity | Cell culture, mice | [64] | |
| Serum | Tyrosinase-related-protein-2 | Spectrofluorometry | Cell culture | [80] | |
| pH-gradient | Neutrophils | Piceatannol | HPLC | Cell culture, mice | [91] |
| Extrusion | Cell culture (MDA-MB-231 cells, MSCs, hESCs, HUVECs) | Different porphyrins | Cell culture | [57] | |
| Cell culture (Raw 264.7 cells) | Catalase | Catalase enzymatic activity | Cell culture, mice | [64] | |
| Freeze-thaw cycles | Cell culture (Raw 264.7 cells) | Catalase | Catalase enzymatic activity | Cell culture, mice | [64] |
| Hypotonic dialysis and extrusion | Cell culture (MDA-MB-231 cells, MSCs, hESCs, HUVECs) | Different porphyrins | Cell culture | [57] | |
| Transfection | Cell culture (HEK293T cells) | miR-21 sponge | RT-qPCR | Cell culture, rats | [92] |
| Cell culture (HEK293T cells) | miR-199a-3p | RT-qPCR | Mice | [11] | |
| Cell culture (HEK293T cells) | miR-199a-3p | RT-qPCR | Cell culture | [93] | |
| Cell culture (HEK293T cells) | Cre recombinase | RT-qPCR | Cell culture | [94] | |
| Cell culture (IC21 cells) | Tripeptidyl-peptidase-1 | Enzymatic activity | Cell culture, mice | [82] | |
| Cell culture (Normal intestinal fetal human cells) | miRNA-128-3p | qPCR | Cell culture, mice with colon tumor xenograft | [77] | |
| Cell culture (MSCs) | Anti-miR-222/223 | Mice with breast tumor xenografts | [95] | ||
| Cell culture (4T1, SKBR3, HepG2 cells) | Anti-miR-21 | Fluorescence microscopy | Cell culture | [96] | |
| TAMEL | Cell culture (HEK293T cells) | RNA | Cell culture | [97] | |
| ExPLORs | Cell culture (HEK293T cells) | Recombinant luciferase | Luciferase activity | Cell culture, mice | [48] |
| Administration Route | Experimental Model | Source of EVs | Therapeutic Cargo | Target Tissues | Reference |
|---|---|---|---|---|---|
| Intravenous | Mice | Plasma | Liver | [151] | |
| Serum | miR-124 | CNS | [152] | ||
| Serum | Lung | [118] | |||
| Serum | Systemic effects | [153] | |||
| Mice serum, supernatant of cultured myotubes | miR-21 | Kidney | [154] | ||
| Blood | Dopamine | CNS | [36] | ||
| Urine | Klotho | Kidney | [116] | ||
| Milk | Liver, spleen, heart, lungs | [145] | |||
| MSC | Paclitaxel | Subcutaneous tumors and distant metastases | [155] | ||
| MSC | Heart | [111] | |||
| MSC | Liver | [156] | |||
| MSC | miR-210 | Brain | [59] | ||
| MSC | miR-let7 | Atherosclerotic plaque | [157] | ||
| MSC | miR-125b | Heart | [158] | ||
| MSC | Bone marrow | [159] | |||
| MSC | CNS | [160] | |||
| MSC, liver stem cells | Subcutaneous tumor | [99] | |||
| AdSC | miR-199ª | Orthotopic tumor | [161] | ||
| AdSC | CNS | [105] | |||
| AdSC | miR-17 | Liver | [114] | ||
| AdSC | Skin | [138,162] | |||
| Bone marrow stromal cells | Liver, lungs, bone | [163] | |||
| HEK293T cells | Anti-miR-214 | Subcutaneous tumor | [89] | ||
| HEK293T cells | Curcumin, miR-143a | Tumor cells | [164] | ||
| HEK293T cells | miR-199a-3p | Subcutaneous tumor | [165] | ||
| HEK293 cells | Mammary tumor | [166] | |||
| Dendritic cells | Spleen | [167] | |||
| Dendritic cells | siRNA | Brain | [107] | ||
| Immature dendritic cells | Doxorubicin | Mammary tumor | [168] | ||
| Mouse brain endothelial cells | miR-126 | CNS | [124] | ||
| Endothelial colony forming cells | miR-486-5p | Kidney | [169] | ||
| Gastric epithelial cells | Aorta | [170] | |||
| Neural primary stem cells | CNS | [171] | |||
| BMD2a cells | Lungs, liver, spleen, brain | [172] | |||
| Liver | miR-130a-3p | Systemic effects | [173] | ||
| Schwann cells | Peripheral nerves | [117] | |||
| Astrocytes | CNS | [174] | |||
| Microglial cells | miR-124-3p | CNS | [122] | ||
| Breast cancer cells | miR-126 | Lung cancer cells | [125] | ||
| Tumor-cell exocytosed-exosome biomimetic porous silicon nanoparticle | Doxorubicin | Tumor cells | [126] | ||
| Gastric cancer cells | Blood myeloid-derived suppressor cells | [175] | |||
| Pancreas carcinoma cells | Liver, spleen, lungs | [176] | |||
| Macrophages | Brain-derived neurotrophic factor | CNS | [177] | ||
| L929 cells | Methotrexate | Glioblastoma tissue | [178] | ||
| Ginger roots | siRNA | Subcutaneous tumor | [42] | ||
| Rats | Serum | CNS | [108] | ||
| MSC | miR-544 | CNS | [102] | ||
| MSC | CC chemokine receptor type 2 | CNS | [179] | ||
| MSC | CNS | [180] | |||
| MSC | CNS | [181] | |||
| MSC | miR-17-92 cluster | CNS | [104] | ||
| MSC | Pulmonary vasculature | [109] | |||
| MSC | Colon | [115] | |||
| MSC | Vein graft | [182] | |||
| MSC | Heart | [183] | |||
| MSC | Lungs | [112] | |||
| MSC | miR-29b | Spinal cord | [184] | ||
| MSC | Pancreas | [113] | |||
| MSC | Carotid artery | [110] | |||
| MSC | Bone | [185] | |||
| AdSC | Penile tissue | [186] | |||
| AdSC | miR-126 | CNS | [187] | ||
| AdSC | Brain, spleen | [188] | |||
| AdSC | Systemic effects | [189] | |||
| AdSC | CNS | [190] | |||
| Human urinary stem cells | miR-26a | CNS | [40] | ||
| Human fetal amniotic fluid stem cells | Heart | [191] | |||
| Cardiac progenitor cells | miR-146a | Heart | [119] | ||
| Cardiac progenitor cells | Cardiomyocytes | [120] | |||
| Cardiac stem cells | Heart | [121] | |||
| Human iPSCs | Bone | [192] | |||
| Urinary stem cells | Kidney | [193] | |||
| Aortic adventitial fibroblasts | miR-155-5p | Aorta and mesenteric artery | [194] | ||
| HEK293T cells | miR-21 antisense | CNS | [106] | ||
| HEK293T cells | siRNA | CNS | [195] | ||
| Human renal tubular cells | Kidney | [196] | |||
| Renal cells | Kidney | [197] | |||
| Patients with COVID-19 | MSC | Systemic effects | [198] | ||
| Monkeys | MSC | CNS | [199] | ||
| Intravenous (in utero) | Ovine fetuses | MSC | Brain | [200] | |
| Intranasal | Mice | MSC | CNS | [201,202,203] | |
| AdSC | CNS | [105] | |||
| ESC | Curcumin | CNS | [204] | ||
| Astrocytes | siRNA | Microglia | [88] | ||
| Amnion epithelial cells | Lungs | [205] | |||
| Macrophages | Lungs | [135,206] | |||
| EL4, 3T3L1, 4T1, CT26 and A20 cells | Curcumin, JSI-124 | CNS | [132] | ||
| Bronchoalveolar lavage fluid | Lungs, systemic effects | [136,207] | |||
| Rats | MSC | CNS | [128,130,131,208] | ||
| MSC | siRNA | CNS | [129] | ||
| C6 Glioma cells | Glioma | [133] | |||
| Human teeth stem cells | CNS | [134] | |||
| Intraperitoneal | Mice | Liver stem cells, MSC | Subcutaneous tumor | [99] | |
| Intramyocardial | Rats | MSC | Heart | [147] | |
| Subcutaneous | Mice | AdSC | Skin | [138] | |
| Echinostoma caproni | Systemic immune response | [137] | |||
| Intramuscular | Mice | Primary mouse satellite cells | miR-29 | Kidney, muscle | [140] |
| Cardiac stem cells | Muscle | [141] | |||
| Human iPSCs | Muscle | [142] | |||
| Chicken | Serum | Systemic immune response | [139] | ||
| Intrathecal | Rats | MSC | Peripheral nerves | [148] | |
| Oral | Mice | Bovine milk | Liver, spleen, heart, lungs, kidney | [144] | |
| Intraocular | Rabbit | MSC | Retina | [146] | |
| Subconjunctival | Rabbit | MSC | Retina | [146] |
| EVs | Disease or Condition | Route of Administration | NCT Number |
|---|---|---|---|
| Plant EVs loaded with curcumin | Colon cancer | Oral | NCT01294072 |
| MSCs EVs loaded with KRAS G12D siRNA | Pancreas cancer | Intravenous | NCT03608631 |
| MSCs | Healthy individuals | Inhalation | NCT04313647 |
| MSCs | SARS-CoV-2 | Inhalation | NCT04276987 |
| MSCs | Acute ischemic stroke | Stereotaxic injection | NCT03384433 |
| MSCs | Macular holes | Intravitreous injection | NCT03437759 |
| MSCs | Dystrophic epidermolysis bullosa | Topical | NCT04173650 |
| MSCs | Depression, anxiety, neurodegenerative disorders | Intravenous | NCT04202770 |
| MSCs | Bronchopulmonary dysplasia | Intravenous | NCT03857841 |
| MSCs | Type 1 diabetes mellitus | Intravenous | NCT02138331 |
| MSCs | SARS-CoV-2 | Inhalation | NCT04491240 |
| MSCs | Alzheimer disease | Intranasal | NCT04388982 |
| MSCs | SARS-CoV-2 | Intravenous | NCT04493242 |
| AdSCs | Periodontitis | Local injection | NCT04270006 |
| Plasma | Cutaneous wounds | Topical | NCT02565264 |
| Not specified | Craniofacial neuralgia | Epineural injection, intravenous | NCT04202783 |
| Not specified | Acute myocardial infarction | Intracoronary | NCT04327635 |
| Plant | Oral mucositis associated with chemoradiotherapy | Topical | NCT01668849 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jong, B.; Barros, E.R.; Hoenderop, J.G.J.; Rigalli, J.P. Recent Advances in Extracellular Vesicles as Drug Delivery Systems and Their Potential in Precision Medicine. Pharmaceutics 2020, 12, 1006. https://doi.org/10.3390/pharmaceutics12111006
de Jong B, Barros ER, Hoenderop JGJ, Rigalli JP. Recent Advances in Extracellular Vesicles as Drug Delivery Systems and Their Potential in Precision Medicine. Pharmaceutics. 2020; 12(11):1006. https://doi.org/10.3390/pharmaceutics12111006
Chicago/Turabian Stylede Jong, Bart, Eric Raul Barros, Joost G. J. Hoenderop, and Juan Pablo Rigalli. 2020. "Recent Advances in Extracellular Vesicles as Drug Delivery Systems and Their Potential in Precision Medicine" Pharmaceutics 12, no. 11: 1006. https://doi.org/10.3390/pharmaceutics12111006
APA Stylede Jong, B., Barros, E. R., Hoenderop, J. G. J., & Rigalli, J. P. (2020). Recent Advances in Extracellular Vesicles as Drug Delivery Systems and Their Potential in Precision Medicine. Pharmaceutics, 12(11), 1006. https://doi.org/10.3390/pharmaceutics12111006