Hybrid Nanoparticles of Poly (Methyl Methacrylate) and Antimicrobial Quaternary Ammonium Surfactants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CTAB or DODAB Dispersions in Water Solution
2.3. Synthesis of Waterborne PMMA/QACs Nanoparticles (NPs) by Emulsion Polymerization
2.4. Determination of Sizes, Zeta-Potentials, and Polydispersity of PMMA/QAC Dispersions by Dynamic Light Scattering (DLS)
2.5. Visualization and Morphology of PMMA/QAC NPs from Scanning Electron Microscopy (SEM)
2.6. Microorganisms Cultures and Effect of CTAB, DODAB, or PMMA/QAC NPs on Cell Viability in the Presence of the Cationic Amphiphiles Solutions or Dispersions
2.7. Determination of Growth Inhibition Zones by PMMA/QAC NPs
2.8. Determination of QAC Concentration from Halide Microtitration
3. Results and Discussion
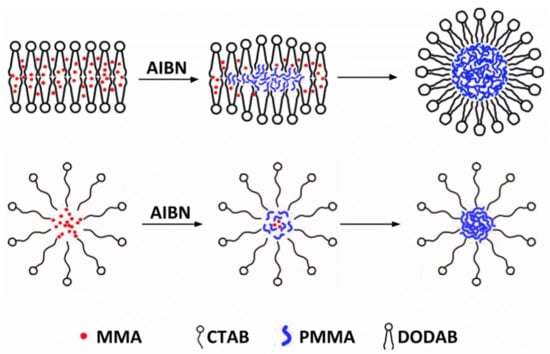
3.1. Synthesis of PMMA/QACs NPs by Emulsion Polymerization and their Physical Characterization from SEM and DLS
3.2. Effects of MMA Concentration, QAC Concentration, and Initiator Type on Physico-Chemical Properties of PMMA/QAC NPs Obtained by Emulsion Polymerization
3.3. Incorporation of QACs in the PMMA/QAC NPs
3.4. Antibacterial and Antifungal Activity of QACs and PMMA/QAC NPs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arif, U.; Haider, S.; Haider, A.; Khan, N.; Alghyamah, A.A.; Jamila, N.; Khan, M.I.; Almasry, W.A.; Kang, I.-K. Biocompatible Polymers and their Potential Biomedical Applications: A Review. Curr. Pharm. Des. 2019, 25, 3608–3619. [Google Scholar] [CrossRef] [PubMed]
- Shastri, V.P. Non-Degradable Biocompatible Polymers in Medicine: Past, Present and Future. Curr. Pharm. Biotechnol. 2003, 4, 331–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, C.N.; Priya, R.; Swain, S.; Kumar Jena, G.; Panigrahi, K.C.; Ghose, D. Pharmaceutical significance of Eudragit: A review. Future J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. Biomimetic Nanomaterials from the Assembly of Polymers, Lipids, and Surfactants. In Surfactants and Detergents; Dutta, A., Ed.; IntechOpen: London, UK, 2019; Volume 1, ISBN 978-1-78984-661-4. [Google Scholar]
- Fournier, R.L. Basic Transport Phenomena in Biomedical Engineering, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-4398-2670-6. [Google Scholar]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Serrano-Aroca, Á. Poly (3-Hydroxybutyrate-co-3-Hydroxyvalerate): Enhancement Strategies for Advanced Applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef] [Green Version]
- Guan, G.; Azad, M.A.K.; Lin, Y.; Kim, S.W.; Tian, Y.; Liu, G.; Wang, H. Biological Effects and Applications of Chitosan and Chito-Oligosaccharides. Front. Physiol. 2019, 10, 516. [Google Scholar] [CrossRef] [Green Version]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Moohan, J.; Stewart, S.A.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, R.F.; Domínguez-Robles, J. Cellulose Nanofibers and Other Biopolymers for Biomedical Applications. A Review. Appl. Sci. 2020, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef]
- Yoshii, E. Cytotoxic effects of acrylates and methacrylates: Relationships of monomer structures and cytotoxicity. J. Biomed. Mater. Res. 1997, 37, 517–524. [Google Scholar] [CrossRef]
- Hua, C.; Chen, K.; Wang, Z.; Guo, X. Preparation, stability and film properties of cationic polyacrylate latex particles with various substituents on the nitrogen atom. Prog. Org. Coat. 2020, 143, 105628. [Google Scholar] [CrossRef]
- Pereira, E.M.A.; Kosaka, P.M.; Rosa, H.; Vieira, D.B.; Kawano, Y.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Hybrid Materials from Intermolecular Associations between Cationic Lipid and Polymers. J. Phys. Chem. B 2008, 112, 9301–9310. [Google Scholar] [CrossRef]
- Melo, L.D.; Palombo, R.R.; Petri, D.F.S.; Bruns, M.; Pereira, E.M.A.; Carmona-Ribeiro, A.M. Structure–Activity Relationship for Quaternary Ammonium Compounds Hybridized with Poly(methyl methacrylate). ACS Appl. Mater. Interfaces 2011, 3, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Sanches, L.M.; Petri, D.F.S.; de Melo Carrasco, L.D.; Carmona-Ribeiro, A.M. The antimicrobial activity of free and immobilized poly (diallyldimethylammonium) chloride in nanoparticles of poly (methylmethacrylate). J. Nanobiotechnol. 2015, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Galvão, C.N.; Sanches, L.M.; Mathiazzi, B.I.; Ribeiro, R.T.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Antimicrobial Coatings from Hybrid Nanoparticles of Biocompatible and Antimicrobial Polymers. Int. J. Mol. Sci. 2018, 19, 2965. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, R.T.; Galvão, C.N.; Betancourt, Y.P.; Mathiazzi, B.I.; Carmona-Ribeiro, A.M. Microbicidal Dispersions and Coatings from Hybrid Nanoparticles of Poly (Methyl Methacrylate), Poly (Diallyl Dimethyl Ammonium) Chloride, Lipids, and Surfactants. Int. J. Mol. Sci. 2019, 20, 6150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lincopan, N.; Espíndola, N.M.; Vaz, A.J.; Carmona-Ribeiro, A.M. Cationic supported lipid bilayers for antigen presentation. Int. J. Pharm. 2007, 340, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Betancourt, Y.; Távora, B.D.C.L.F.; Colombini, M.; Faquim-Mauro, E.L.; Carmona-Ribeiro, A.M. Simple Nanoparticles from the Assembly of Cationic Polymer and Antigen as Immunoadjuvants. Vaccines 2020, 8, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efron, N. Contact Lens Practice E-Book; Elsevier Health Sciences: Amsterdam, The Netherland, 2016; ISBN 978-0-7020-6661-0. [Google Scholar]
- Naves, A.F.; Palombo, R.R.; Carrasco, L.D.M.; Carmona-Ribeiro, A.M. Antimicrobial Particles from Emulsion Polymerization of Methyl Methacrylate in the Presence of Quaternary Ammonium Surfactants. Langmuir 2013, 29, 9677–9684. [Google Scholar] [CrossRef]
- Ahlström, B.; Chelminska-Bertilsson, M.; Thompson, R.A.; Edebo, L. Submicellar complexes may initiate the fungicidal effects of cationic amphiphilic compounds on Candida albicans. Antimicrob. Agents Chemother. 1997, 41, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Martins, L.M.S.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Cationic Vesicles as Bactericides. Langmuir 1997, 13, 5583–5587. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Vieira, D.B.; Lincopan, N. Cationic Surfactants and Lipids as Anti-Infective Agents. Anti-Infect. Agents Med. Chem. 2006, 5, 33–51. [Google Scholar] [CrossRef]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Cationic lipids and surfactants as antifungal agents: Mode of action. J. Antimicrob. Chemother. 2006, 58, 760–767. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 2015; ISBN 978-0-08-092363-5. [Google Scholar]
- Carmona-Ribeiro, A.M. Synthetic amphiphile vesicles. Chem. Soc. Rev. 1992, 21, 209–214. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. Lipid Bilayer Fragments and Disks in Drug Delivery. Curr. Med. Chem. 2006, 13, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. The Versatile Dioctadecyldimethylammonium Bromide. In Application and Characterization of Surfactants; Najjar, R., Ed.; IntechOpen: Rijeka, Croatia, 2017; Volume 1, pp. 157–181. ISBN 978-953-51-3325-4. [Google Scholar]
- Tapias, G.N.; Sicchierolli, S.M.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Interactions between Cationic Vesicles and Escherichia coli. Langmuir 1994, 10, 3461–3465. [Google Scholar] [CrossRef]
- Sicchierolli, S.M.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Bacteria Flocculation and Death by Cationic Vesicles. Langmuir 1995, 11, 2991–2995. [Google Scholar] [CrossRef]
- Campanhã, M.T.N.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Interactions between cationic liposomes and bacteria: The physical-chemistry of the bactericidal action. J. Lipid Res. 1999, 40, 1495–1500. [Google Scholar] [PubMed]
- Campanhã, M.T.N.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Interactions between Cationic Vesicles and Candida albicans. J. Phys. Chem. B 2001, 105, 8230–8236. [Google Scholar] [CrossRef]
- Mamizuka, E.M.; Carmona-Ribeiro, A.M. Cationic Liposomes as Antimicrobial Agents. In Communicating Current Research and Educational Topics and Trends in Applied Microbiology; A. Méndez Vila: Badajoz, Spain, 2007; Volume 2, pp. 636–647, ISBN 13: 978-84-611-9423-0. [Google Scholar]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Cationic nanoparticles for delivery of amphotericin B: Preparation, characterization and activity in vitro. J. Nanobiotechnol. 2008, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, L.A.; Carmona-Ribeiro, A.M. Interactions between Cationic Vesicles and Serum Proteins. Langmuir 1998, 14, 6077–6081. [Google Scholar] [CrossRef]
- Xavier, G.R.S.; Carmona-Ribeiro, A.M. Cationic Biomimetic Particles of Polystyrene/Cationic Bilayer/Gramicidin for Optimal Bactericidal Activity. Nanomaterials 2017, 7, 422. [Google Scholar] [CrossRef] [Green Version]
- Ragioto, D.A.; Carrasco, L.D.; Carmona-Ribeiro, A.M. Novel gramicidin formulations in cationic lipid as broad-spectrum microbicidal agents. Int. J. Nanomed. 2014, 9, 3183–3192. [Google Scholar]
- Kikuchi, I.S.; Carmona-Ribeiro, A.M. Interactions between DNA and Synthetic Cationic Liposomes. J. Phys. Chem. B 2000, 104, 2829–2835. [Google Scholar] [CrossRef]
- Rosa, H.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Interactions between Bacteriophage DNA and Cationic Biomimetic Particles. J. Phys. Chem. B 2008, 112, 16422–16430. [Google Scholar] [CrossRef]
- Rozenfeld, J.H.K.; Silva, S.R.; Ranéia, P.A.; Faquim-Mauro, E.; Carmona-Ribeiro, A.M. Stable assemblies of cationic bilayer fragments and CpG oligonucleotide with enhanced immunoadjuvant activity in vivo. J. Controll. Release 2012, 160, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Bilayer-Forming Synthetic Lipids: Drugs or Carriers? Curr. Med. Chem. 2003, 10, 2425–2446. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Synthetic Bilayer Fragments for Solubilization of Amphotericin B. J. Colloid Interface Sci. 2001, 244, 427–431. [Google Scholar] [CrossRef]
- Carvalho, C.A.; Olivares-Ortega, C.; Soto-Arriaza, M.A.; Carmona-Ribeiro, A.M. Interaction of gramicidin with DPPC/DODAB bilayer fragments. Biochim. Biophys. Acta BBA-Biomembr. 2012, 1818, 3064–3071. [Google Scholar] [CrossRef]
- Melo, L.D.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Antimicrobial Particles from Cationic Lipid and Polyelectrolytes. Langmuir 2010, 26, 12300–12306. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Novel Formulations for Antimicrobial Peptides. Int. J. Mol. Sci. 2014, 15, 18040–18083. [Google Scholar] [CrossRef] [Green Version]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic Antimicrobial Polymers and Their Assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [Green Version]
- Schales, O.; Schales, S. A simple and accurate method for the determination of chloride in biological fluids. J. Biol. Chem. 1941, 140, 879–884. [Google Scholar]
- Carmona-Ribeiro, A.M. Preparation and Characterization of Biomimetic Nanoparticles for Drug Delivery. In Nanoparticles in Biology and Medicine; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 283–294. ISBN 978-1-61779-952-5. [Google Scholar]
- Grabowski, E.; Morrison, I. Particle size distribution from analysis of quasi-elastic light scattering data. In Measurement of Suspended Particles by Quasi-elastic Light Scattering; John Wiley & Sons: New York, NY, USA, 1983; Volume 21, pp. 199–236. [Google Scholar]
- Lincopan, N.; Santana, M.R.; Faquim-Mauro, E.; da Costa, M.H.B.; Carmona-Ribeiro, A.M. Silica-based cationic bilayers as immunoadjuvants. BMC Biotechnol. 2009, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, L.D.; de, M.; Bertolucci, R.J.; Ribeiro, R.T.; Sampaio, J.L.M.; Carmona-Ribeiro, A.M. Cationic Nanostructures against Foodborne Pathogens. Front. Microbiol. 2016, 7, 1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, P.S.; Dasannacharya, B.A.; Kelkar, V.K.; Manohar, C.; Srinivasa Rao, K.; Valaulikar, B.S. Shapes and sizes of micelles in CTAB solutions. Phys. B Condens. Matter 1991, 174, 196–199. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Chaimovich, H. Preparation and characterization of large dioctadecyldimethylammonium chloride liposomes and comparison with small sonicated vesicles. Biochim. Biophys. Acta BBA-Biomembr. 1983, 733, 172–179. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Yoshida, L.S.; Chaimovich, H. Salt effects on the stability of dioctadecyldimethylammonium chloride and sodium dihexadecyl phosphate vesicles. J. Phys. Chem. 1985, 89, 2928–2933. [Google Scholar] [CrossRef]
- Lo, C.T.; Van Tassel, P.R.; Saltzman, W.M. Simultaneous release of multiple molecules from poly(lactide-co-glycolide) nanoparticles assembled onto medical devices. Biomaterials 2009, 30, 4889–4897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Date, P.; Ottoor, D. pH Dependent Controlled Release of CTAB Incorporated Dipyridamole Drug from Agar-Based Hydrogel. Polym.-Plast. Technol. Eng. 2016, 55, 403–413. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef]
- Sun, M.; Ding, Z.; Wang, H.; Yu, G.; Li, B.; Li, M.; Zhen, M. Antifungal effects of BiOBr nanosheets carrying surfactant cetyltrimethylammonium bromide. J. Biomed. Res. 2018, 32, 380–388. [Google Scholar]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Cheng, C.J.; Tietjen, G.T.; Saucier-Sawyer, J.K.; Saltzman, W.M. A holistic approach to targeting disease with polymeric nanoparticles. Nat. Rev. Drug Discov. 2015, 14, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Otto, M. Staphylococcus epidermidis—The “accidental” pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukushima, K.; Tan, J.P.K.; Korevaar, P.A.; Yang, Y.Y.; Pitera, J.; Nelson, A.; Maune, H.; Coady, D.J.; Frommer, J.E.; Engler, A.C.; et al. Broad-Spectrum Antimicrobial Supramolecular Assemblies with Distinctive Size and Shape. ACS Nano 2012, 6, 9191–9199. [Google Scholar] [CrossRef] [PubMed]
- Carmona Ribeiro, A.M.; Carrasco, L.D.M. Fungicidal assemblies and their mode of action. OA Biotechnol. 2013, 2, 25. [Google Scholar] [CrossRef] [Green Version]









| NPs | D (nm) | Dz (nm) |
|---|---|---|
| PMMA/DODAB | 56 ± 7 | 75 ± 1 |
| PMMA/CTAB | 85 ± 11 | 81 ± 1 |
| QAC | [MMA] /M | Dz /nm | ζ /mV | P | Solid Contents /mg·mL−1 | Conversion /% | Np /mL−1 | Aggregates |
|---|---|---|---|---|---|---|---|---|
| DODAB | 0.1 | 101 ± 1 | +20 ± 2 | 0.355 ± 0.002 | 0.0051 ± 0.0001 | 51 | 8.35 × 1012 | No |
| 0.2 | 52 ± 1 | +20 ± 1 | 0.265 ± 0.003 | 0.0081 ± 0.0001 | 40 | 9.52 × 1013 | No | |
| 0.3 | 73 ± 1 | +27 ± 1 | 0.038 ± 0.010 | 0.0183 ± 0.0009 | 61 | 7.76 × 1013 | No | |
| 0.4 | 75 ± 1 | +49 ± 5 | 0.037 ± 0.005 | 0.0317 ± 0.0001 | 79 | 1.26 × 1014 | No | |
| 0.7 | 94 ± 1 | +26 ± 2 | 0.033 ± 0.009 | 0.0479 ± 0.0008 | 68 | 1.04 × 1014 | Yes | |
| 0.9 | 103 ± 1 | +38 ± 2 | 0.013 ± 0.004 | 0.0697 ± 0.0001 | 77 | 1.07 × 1014 | Yes | |
| 1.0 | 109 ± 1 | +33 ± 1 | 0.020 ± 0.010 | 0.0781 ± 0.0005 | 78 | 1.02 × 1014 | Yes | |
| CTAB | 0.1 | 916 ± 56 | +18 ± 1 | 0.477 ± 0.042 | 0.0021 ± 0.0004 | 20 | 4.43 × 109 | No |
| 0.2 | 40 ± 1 | +20 ± 1 | 0.291 ± 0.003 | 0.0105 ± 0.0004 | 53 | 2.82 × 1014 | No | |
| 0.3 | 70 ± 1 | +17 ± 1 | 0.078 ± 0.009 | 0.0197 ± 0.0019 | 65 | 9.52 × 1013 | No | |
| 0.4 | 81 ± 1 | +23 ± 1 | 0.041 ± 0.008 | 0.0320 ± 0.0019 | 80 | 1.01 × 1014 | No | |
| 0.7 | 100 ± 1 | +20 ± 1 | 0.032 ± 0.008 | 0.0363 ± 0.0014 | 52 | 6.10 × 1013 | Yes | |
| 0.9 | 121 ± 1 | +26 ± 1 | 0.012± 0.004 | 0.0692 ± 0.0013 | 77 | 6.52 × 1013 | Yes | |
| 1.0 | 121 ± 1 | +17 ± 1 | 0.046 ± 0.010 | 0.0702 ± 0.0005 | 70 | 6.52 × 1013 | Yes |
| QAC | [QAC] /mM | Dz /nm | ζ /mV | P | Solid Contents /mg·mL−1 | Conversion /% | Np /mL−1 |
|---|---|---|---|---|---|---|---|
| DODAB | 0.3 | 99 ± 1 | +50 ± 2 | 0.035 ± 0.009 | 0.0303 ± 0.0003 | 76 | 5.24 × 1013 |
| 0.5 | 93 ± 1 | +44 ± 2 | 0.045 ± 0.014 | 0.0300 ± 0.0004 | 75 | 6.21 × 1013 | |
| 1.0 | 85 ± 1 | +33 ± 2 | 0.028 ± 0.008 | 0.0303 ± 0.0006 | 76 | 8.08 × 1013 | |
| 2.0 | 75 ± 1 | +49 ± 5 | 0.037 ± 0.005 | 0.0317 ± 0.0001 | 79 | 1.26 × 1014 | |
| 4.0 | 69 ± 1 | +31 ± 1 | 0.072 ± 0.008 | 0.0349 ± 0.0006 | 87 | 1.79 × 1014 | |
| 5.0 | 62 ± 1 | +29 ± 2 | 0.068 ± 0.010 | 0.0333 ± 0.0006 | 83 | 2.31 × 1014 | |
| 8.0 | 58 ± 1 | +32 ± 2 | 0.098 ± 0.010 | 0.0365 ± 0.0008 | 91 | 3.04 × 1014 | |
| 10.0 | 59 ± 1 | +35 ± 3 | 0.123 ± 0.007 | 0.0364 ± 0.0021 | 91 | 2.92 × 1014 | |
| CTAB | 0.3 | 126 ± 1 | +15 ± 1 | 0.055 ± 0.013 | 0.0261 ± 0.0001 | 65 | 2.16 × 1013 |
| 0.5 | 115 ± 1 | +15 ± 1 | 0.069 ± 0.016 | 0.0274 ± 0.0001 | 68 | 3.02 × 1013 | |
| 1.0 | 103 ± 1 | +27 ± 2 | 0.069 ± 0.013 | 0.0319 ± 0.0005 | 80 | 4.86 × 1013 | |
| 2.0 | 81 ± 1 | +23 ± 1 | 0.041 ± 0.008 | 0.0320 ± 0.0019 | 80 | 1.01 × 1014 | |
| 4.0 | 67 ± 1 | +24 ± 1 | 0.070 ± 0.011 | 0.0282 ± 0.0003 | 70 | 1.55 × 1014 | |
| 5.0 | 67 ± 1 | +31 ± 2 | 0.052 ± 0.012 | 0.0234 ± 0.0006 | 58 | 1.32 × 1014 | |
| 8.0 | 60 ± 1 | +34 ± 1 | 0.095 ± 0.011 | 0.0320 ± 0.0002 | 80 | 2.50 × 1014 | |
| 10.0 | 57 ± 1 | +41 ± 2 | 0.092 ± 0.008 | 0.0308 ± 0.0001 | 77 | 2.72 × 1014 |
| NPs | Initiator | Dz/nm | ζ/mV | P |
|---|---|---|---|---|
| PMMA/DODAB | KPS 1 | 1260 ± 43 | −10 ± 1 | 0.370 |
| AIBN | 89 ± 1 | +45 ± 2 | 0.027 ± 0.010 | |
| PMMA/CTAB | KPS 1 | 395 ± 5 | −38 ± 1 | 0.262 |
| AIBN | 96 ± 1 | +23 ± 1 | 0.033 ± 0.012 |
| Dispersion or Solution | [QAC]/mM | |
|---|---|---|
| Before Dialysis | After Dialysis | |
| CTAB dispersion in water | 2.5 ± 0.1 | 0.1 ± 0.1 |
| DODAB bilayer fragments in water | 2.3 ± 0.1 | 2.0 ± 0.1 |
| NaCl water solution | 1.2 ± 0.1 | 0.2 ± 0.1 |
| Supernatant of PMMA/DODAB dispersion | 2.0 ± 0.1 | 1.3 ± 0.1 1 |
| Supernatant of PMMA/CTAB dispersion | 2.0 ± 0.1 | 0.5 ± 0.1 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathiazzi, B.I.; Carmona-Ribeiro, A.M. Hybrid Nanoparticles of Poly (Methyl Methacrylate) and Antimicrobial Quaternary Ammonium Surfactants. Pharmaceutics 2020, 12, 340. https://doi.org/10.3390/pharmaceutics12040340
Mathiazzi BI, Carmona-Ribeiro AM. Hybrid Nanoparticles of Poly (Methyl Methacrylate) and Antimicrobial Quaternary Ammonium Surfactants. Pharmaceutics. 2020; 12(4):340. https://doi.org/10.3390/pharmaceutics12040340
Chicago/Turabian StyleMathiazzi, Beatriz Ideriha, and Ana Maria Carmona-Ribeiro. 2020. "Hybrid Nanoparticles of Poly (Methyl Methacrylate) and Antimicrobial Quaternary Ammonium Surfactants" Pharmaceutics 12, no. 4: 340. https://doi.org/10.3390/pharmaceutics12040340
APA StyleMathiazzi, B. I., & Carmona-Ribeiro, A. M. (2020). Hybrid Nanoparticles of Poly (Methyl Methacrylate) and Antimicrobial Quaternary Ammonium Surfactants. Pharmaceutics, 12(4), 340. https://doi.org/10.3390/pharmaceutics12040340