Assessment of Residual Solvent and Drug in PLGA Microspheres by Derivative Thermogravimetry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLGA Microspheres Using Ethyl Formate
2.3. Preparation of PLGA Microspheres Using Methylene Chloride
2.4. Particle Size Analysis
2.5. Scanning Electron Microscopy (SEM)
2.6. Encapsulation Efficiency of Progesterone
2.7. Gas Chromatography (GC)
2.8. Thermogravimetry (TG)
2.9. Derivative Thermogravimetry (DTG)
3. Results and discussion
3.1. Characterization of PLGA Microspheres Prepared Using Ethyl Formate
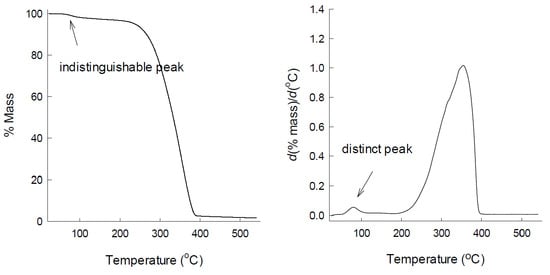
3.2. Comparison of TG and DTG Curves of PLGA Microspheres
3.3. Measurement of Residual Solvents by DTG
3.4. Effect of Progestrone upon Thermal Behavior of Ethyl Formate
3.5. Thermal Drug Analysis—Contemporary TG Approaches vs. our DTG Technique
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Prakapenka, A.V.; Bimonte-Nelson, H.A.; Sirianni, R.W. Engineering poly(lactic-co-glycolic acid) (PLGA) micro- and nano-carriers for controlled delivery of 17β-estradiol. Ann. Biomed. Eng. 2017, 45, 1697–1709. [Google Scholar] [CrossRef]
- Wu, L.; Janagam, D.R.; Mandrell, T.D.; Johnson, J.R.; Lowe, T.L. Long-acting injectable hormonal dosage forms for contraception. Pharm. Res. 2015, 32, 2180–2191. [Google Scholar] [CrossRef]
- Terukina, T.; Naito, Y.; Tagami, T.; Morikawa, Y.; Henmi, Y.; Prananingrum, W.; Ichikawa, T.; Ozeki, T. The effect of the release behavior of simvastatin from different PLGAparticles on bone regenerationin vitroandin vivo: Comparison of simvastatin-loaded PLGA microspheres and nanospheres. J. Drug Deliv. Sci. Technol. 2016, 33, 136–142. [Google Scholar] [CrossRef]
- Haque, S.; Boyd, B.J.; McIntosh, M.P.; Pouton, C.W.; Kaminskas, L.M.; Whittaker, M. Suggested procedures for the reproducible synthesis of poly(d,l-lactide-co-glycolide) nanoparticles using the emulsification solvent diffusion platform. Curr. Nanosci. 2018, 14, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Mensah, R.A.; Kirton, S.B.; Cook, M.T.; Styliari, I.D.; Hutter, V.; Chau, D. Optimising poly(lactic-co-glycolic acid) microparticle fabrication using a Taguchi orthogonal array design-of-experiment approach. PLOS One 2019, 14, e0222858. [Google Scholar] [CrossRef] [PubMed]
- Ekanem, E.E.; Nabavi, S.A.; Vladisavljević, G.T.; Gu, S. Structured biodegradable polymeric microparticles for drug delivery produced using flow focusing glass microfluidic devices. ACS Appl. Mater. Interfaces 2015, 7, 23132–23143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Xue, J.; Jia, L.; Du, Q.; Niu, J.; Zhang, D. Surface-modified PLGA nanoparticles with chitosan for oral delivery of tolbutamide. Colloids Surf. B Biointerfaces 2018, 161, 67–72. [Google Scholar] [CrossRef]
- Miele, D.; Catenacci, L.; Sorrenti, M.; Rossi, S.; Sandri, G.; Malavasi, L.; Dacarro, G.; Ferrari, F.; Bonferoni, M.C. Chitosan oleate coated poly lactic-glycolic acid (PLGA) nanoparticles versus chitosan oleate self-assembled polymeric micelles, loaded with resveratrol. Mar. drugs 2019, 17, 515. [Google Scholar] [CrossRef] [Green Version]
- Noviendri, D.; Jaswir, I.; Taher, M.; Mohamed, F.; Salleh, H.M.; Noorbatcha, I.A.; Octavianti, F.; Lestari, W.; Hendri, R.; Ahmad, H.; et al. Fabrication of fucoxanthin-loaded microsphere (F-LM) by two steps double-emulsion solvent evaporation method and characterization of fucoxanthin before and after microencapsulation. J. Oleo. Sci. 2016, 65, 641–653. [Google Scholar] [CrossRef] [Green Version]
- Tao, C.; Huang, J.; Lu, Y.; Zou, H.; He, X.; Chen, Y.; Zhong, Y. Development and characterization of GRGDSPC-modified poly(lactide-co-glycolide acid) porous microspheres incorporated with protein-loaded chitosan microspheres for bone tissue engineering. Colloids Surf. B Biointerfaces 2014, 122, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, N.; Yonebayashi, K.; Okazaki, M.; Nishiyama, S.; Nishi, T.; Osaka, A.; Watanabe, C. Characterization of soil organic matter with different degrees of humification using evolved gas analysis-mass spectrometry. Talanta 2016, 155, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Song, F.; Zhang, J.; Liu, S.; Xing, B.; Bai, Y. Pyrolysis characteristics of soil humic substances using TG-FTIR-MS combined with kinetic models. Sci. Total Environ. 2020, 698, 134237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, G.; Wang, Q.; Cui, Z.; Wang, L. Pyrolysis characteristics, kinetics, and evolved gas determination of chrome-tanned sludge by thermogravimetry-Fourier-transform infrared spectroscopy and pyrolysis gas chromatography-mass spectrometry. Waste Manag. 2019, 93, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Risoluti, R.; Materazzi, S. Mass spectrometry for evolved gas analysis: An update. Appl. Spectros. Rev. 2019, 54, 87–116. [Google Scholar] [CrossRef]
- Kukułka, W.; Wenelska, K.; Baca, M.; Chen, X.; Mijowska, E. From hollow to solid carbon spheres: Time-dependent facile synthesis. Nanomaterials (Basel) 2018, 8, 861. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Du, W.; Shi, P.; Shangguan, J.; Liu, S.; Zhou, C.; Chen, P.; Zhang, Q.; Fan, H. Mechanistic and kinetic analysis of Na2SO4-modified laterite decomposition by thermogravimetry coupled with mass spectrometry. PLOS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Kaitsuka, Y.; Hayashi, N.; Shimokawa, T.; Togawa, E.; Goto, H. Synthesis of polyaniline (PANI) in nano-reaction field of cellulose nanofiber (CNF), and carbonization. Polymers (Basel) 2016, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.G.; Silva Júnior, W.F.; Veiga Junior, V.F.; Lima, Á.A.; Lima, E.S. Physicochemical characterization and biological activities of the triterpenic mixture α,β-amyrenone. Molecules 2017, 22, 298. [Google Scholar] [CrossRef] [Green Version]
- Guarino, V.; Causa, F.; Taddei, P.; di Foggia, M.; Ciapetti, G.; Martini, D.; Fagnano, C.; Baldini, N.; Ambrosio, L. Polylactic acid fibre-reinforced polycaprolactone scaffolds for bone tissue engineering. Biomaterials 2008, 29, 3662–3670. [Google Scholar] [CrossRef]
- Sivalingam, G.; Vijayalakshmi, S.P.; G. Madras, G. Enzymatic and thermal degradation of poly(ɛ-caprolactone), poly (d,l-lactide), and their blends. Ind. Eng. Chem. Res. 2004, 43, 7702–7709. [Google Scholar] [CrossRef]
- Bragagni, M.; Beneitez, C.; Martín, C.; Hernán Pérez de la Ossa, D.; Mura, P.A.; Gil-Alegre, M.E. Selection of PLA polymers for the development of injectable prilocaine controlled release microparticles: Usefulness of thermal analysis. Int. J. Pharm. 2013, 441, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.D.; Son, S.; Sadiasa, A.; Min, Y.K.; Lee, B.T. Preparation and characterization of PLGA microspheres by the electrospraying method for delivering simvastatin for bone regeneration. Int. J. Pharm. 2013, 443, 87–94. [Google Scholar] [CrossRef]
- Shim, H.; Sah, H. Qualification of non-halogenated organic solvents applied to microsphere manufacturing process. Pharmaceutics 2020, 12, 425. [Google Scholar] [CrossRef]
- Yerragunta, B.; Jogala, S.; Chinnala, K.M.; Aukunuru, J. Development of a novel 3-month drug releasing risperidone microspheres. J. Pharm. Bioallied. Sci. 2015, 7, 37–44. [Google Scholar] [PubMed]
- Saliba, J.B.; da Silva-Cunha Jr, A.; da Silva, G.R.; Yoshida, M.I.; Mansur, A.A.P.; Mansur, H.S. Characterization and in vitro release of cyclosporine-A from poly(D,L-lactide-co-glycolide implants obtained by solvent/extraction evaporation. Química Nova 2012, 35, 723–727. [Google Scholar] [CrossRef]
- Park, C.W.; Lee, H.J.; Oh, D.W.; Kang, J.H.; Han, C.S.; Kim, D.W. Preparation and in vitro/in vivo evaluation of PLGA microspheres containing norquetiapine for long-acting injection. Drug Des. Devel. Ther. 2018, 12, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Busatto, C.; Pesoa, J.; Helbling, I.; Luna, J.; Estenoz, D. Effect of particle size, polydispersity and polymer degradation on progesterone release from PLGA microparticles: Experimental and mathematical modeling. Int. J. Pharm. 2018, 536, 360–369. [Google Scholar] [CrossRef]
- Pu, C.; Wang, Q.; Zhang, H.; Gou, J.; Guo, Y.; Tan, X.; Xie, B.; Yin, N.; He, H.; Zhang, Y.; et al. In vitro-in vivo relationship of amorphous insoluble API (progesterone) in PLGA microspheres. Pharm. Res. 2017, 34, 2787–2797. [Google Scholar] [CrossRef]
- Weldon, C.B.; Tsui, J.H.; Shankarappa, S.A.; Nguyen, V.T.; Ma, M.; Anderson, D.G.; Kohane, D.S. Electrospun drug-eluting sutures for local anesthesia. J. Control. Release 2012, 161, 903–909. [Google Scholar] [CrossRef]
- Javiya, C.; Jonnalagadda, S. Physicochemical characterization of spray-dried PLGA/PEG microspheres, and preliminary assessment of biological response. Drug Dev. Ind. Pharm. 2016, 42, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.; Dixon, D. The influence of solvent processing on polyester bioabsorbable polymers. J. Biomater. Appl. 2012, 26, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Okada, H. One- and three-month release injectable microspheres of the LH-RH superagonist leuprorelin acetate. Adv. Drug Delivery Rev. 1997, 28, 43–70. [Google Scholar] [CrossRef]
- Blasi, P.; Schoubben, A.; Giovagnoli, S.; Perioli, L.; Ricci, M.; Rossi, C. Ketoprofen poly(lactide-co-glycolide) physical interaction. AAPS PharmSciTech. 2007, 8, E78–E85. [Google Scholar] [CrossRef]
- Haseeb, R.; Lau, M.; Sheah, M.; Montagner, F.; Quiram, G.; Palmer, K.; Stefan, M.C.; Rodrigues, D.C. Synthesis and characterization of new chlorhexidine-containing nanoparticles for root canal disinfection. Materials 2016, 9, 452. [Google Scholar] [CrossRef]
- Khan, S.; Chauhan, N.; Yallapu, M.M.; Ebeling, M.C.; Balakrishna, S.; Ellis, R.T.; Thompson, P.A.; Balabathula, P.; Behrman, S.W.; Zafar, N.; et al. Nanoparticle formulation of ormeloxifene for pancreatic cancer. Biomaterials 2015, 53, 731–743. [Google Scholar] [CrossRef] [Green Version]
- Saha, C.; Kaushik, A.; Das, A.; Pal, S.; Majumder, D. Anthracycline drugs on modified surface of quercetin-loaded polymer nanoparticles: A dual drug delivery model for cancer treatment. PLoS ONEe 2016, 11, e0155710. [Google Scholar] [CrossRef]
- Swy, E.R.; Schwartz-Duval, A.S.; Shuboni, D.D.; Latourette, M.T.; Mallet, C.L.; Parys, M.; Cormode, D.P.; Shapiro, E.M. Dual-modality, fluorescent, PLGA encapsulated bismuth nanoparticles for molecular and cellular fluorescence imaging and computed tomography. Nanoscale 2014, 6, 13104–13112. [Google Scholar] [CrossRef] [Green Version]
- Saez, V.; Cerruti, R.; Ramón, J.A.; Santos, E.R.F.; Silva, D.Z.; Pinto, J.C.; Souza Jr, F.G. Quantification of oxaliplatin encapsulated into PLGA microspheres by TGA. Macromol. Symp. 2016, 368, 116–121. [Google Scholar] [CrossRef]
- Nan, K.; Ma, F.; Hou, H.; Freeman, W.R.; Sailor, M.J.; Cheng, L. Porous silicon oxide-PLGA composite microspheres for sustained ocular delivery of daunorubicin. Acta. Biomater. 2014, 10, 3505–3512. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Song, F.; Ju, Y.; Huang, L.; Zhang, L.; Tang, C.; Yang, H.; Huang, C. NAC-loaded electrospun scaffolding system with dual compartments for the osteogenesis of rBMSCs in vitro. Int. J. Nanomedicine 2019, 14, 787–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Property | Ethyl Formate | Methylene Chloride |
|---|---|---|
| Formula | HCOOC2H5 | CH2Cl2 |
| Molecular mass (g/mol) | 74.08 | 88.93 |
| ICH solvent class | 3 | 2 |
| Boiling point (°C) | 54.4 | 40 |
| Density (g/cm3) | 0.92 | 1.33 |
| Solubility in water (g/100 mL) | 10.5 | 1.3 |
| Log P (octanol/water) | 0.23 | 1.25 |
| Progesterone % | To | Te | Tp | Peak Area |
|---|---|---|---|---|
| d(% mass)/d(°C) | ||||
| 0.0 | 50.6 ± 0.5 | 134.4 ± 3.0 | 89.6 ± 0.5 | 0.1241 ± 0.0039 |
| 13.1 | 48.0 ± 0.6 | 121.2 ± 2.6 | 80.5 ± 0.6 | 0.0680 ± 0.0033 |
| 20.7 | 50.1 ± 1.0 | 117.9 ± 2.8 | 81.6 ± 2.4 | 0.0628 ± 0.0032 |
| 27.4 | 50.2 ± 0.5 | 115.2 ± 1.3 | 80.8 ± 0.9 | 0.0545 ± 0.0011 |
| 32.8 | 49.3 ± 0.1 | 116.2 ± 1.1 | 80.0 ± 0.6 | 0.0566 ± 0.0015 |
| d(% mass)/d(min) | ||||
| 0.0 | 50.4 ± 0.9 | 138.8 ± 4.6 | 89.9 ± 1.3 | 2.595 ± 0.081 |
| 13.1 | 48.3 ± 0.3 | 119.1 ± 2.9 | 80.8 ± 0.8 | 1.333 ± 0.073 |
| 20.7 | 50.7 ± 1.7 | 117.0 ± 1.8 | 82.5 ± 1.9 | 1.252 ± 0.061 |
| 27.4 | 50.1 ± 1.0 | 114.7 ± 1.3 | 81.4 ± 1.2 | 1.090 ± 0.017 |
| 32.8 | 49.5 ± 0.5 | 115.0 ± 2.1 | 79.9 ± 0.9 | 1.129 ± 0.007 |
| Assay | Progesterone Content (%) in Microspheres | |
|---|---|---|
| 13.1 | 32.8 | |
| GC | 1.93 ± 0.06 | 1.66 ± 0.06 |
| d(% mass)/d(°C) | 2.13 ± 0.11 | 1.58 ± 0.02 |
| d(% mass)/d(min) | 2.10 ± 0.11 | 1.57 ± 0.02 |
| Assay | Progesterone Content (%) in Microspheres | |
|---|---|---|
| 0 | 18.5 | |
| GC | 3.29 ± 0.28 | 2.77 ± 0.35 |
| DTG, d(% mass)/d(°C) | 3.51 ± 0.20 | 2.60 ± 0.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, H.; Sah, H. Assessment of Residual Solvent and Drug in PLGA Microspheres by Derivative Thermogravimetry. Pharmaceutics 2020, 12, 626. https://doi.org/10.3390/pharmaceutics12070626
Shim H, Sah H. Assessment of Residual Solvent and Drug in PLGA Microspheres by Derivative Thermogravimetry. Pharmaceutics. 2020; 12(7):626. https://doi.org/10.3390/pharmaceutics12070626
Chicago/Turabian StyleShim, Hyunjin, and Hongkee Sah. 2020. "Assessment of Residual Solvent and Drug in PLGA Microspheres by Derivative Thermogravimetry" Pharmaceutics 12, no. 7: 626. https://doi.org/10.3390/pharmaceutics12070626
APA StyleShim, H., & Sah, H. (2020). Assessment of Residual Solvent and Drug in PLGA Microspheres by Derivative Thermogravimetry. Pharmaceutics, 12(7), 626. https://doi.org/10.3390/pharmaceutics12070626