State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis
Abstract
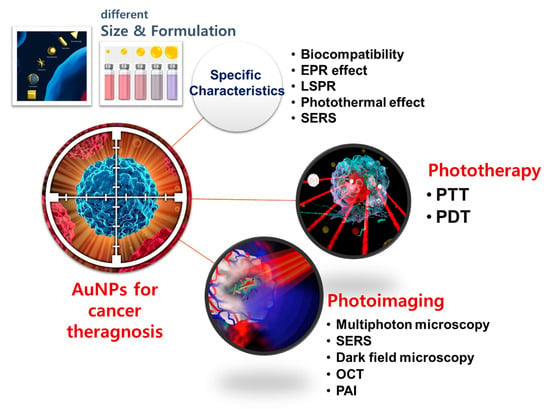
:1. Introduction
2. Key Properties of Gold Nanoparticles (AuNPs) Important for Cancer Theragnosis
2.1. Biocompatibility
2.2. Physicochemical Characteristics
2.2.1. Enhanced Permeability and Retention (EPR) Effect
2.2.2. Localized Surface Plasmon Resonance (LSPR)
2.2.3. Photothermal Effect
2.2.4. Surface Enhanced Raman Scattering (SERS)
3. Phototherapy
3.1. Photothermal Therapy (PTT) with Gold Nanoparticles
3.2. Photodynamic Therapy (PDT) of Gold Nanoparticles
4. Photoimaging
4.1. Surface Enhanced Raman Spectroscopy (SERS)
4.2. Multiphoton Photoluminescence Imaging
4.3. Dark Field Microscopy
4.4. Optical Coherence Tomography (OCT)
4.5. Photoacoustic Imaging (PAI)
5. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Murai, H.; Mitoma, S.; Tokuyama, T.; Motomura, M. Gold Wire for Semiconductor Element Connection and Semiconductor Element Connection Method. U.S. Patent 6,492,593, 10 December 2002. [Google Scholar]
- Bakshi, M.S.; Thakur, P.; Sachar, S.; Banipal, T.S. Synthesis of nanocomposite gold-semiconductor materials by seed-growth method. Mater. Lett. 2007, 61, 3762–3767. [Google Scholar] [CrossRef]
- Sanchez, A.; Abbet, S.; Heiz, U.; Schneider, W.-D.; Häkkinen, H.; Barnett, R.; Landman, U. When gold is not noble: Nanoscale gold catalysts. J. Phys. Chem. A 1999, 103, 9573–9578. [Google Scholar] [CrossRef]
- Claus, P. Heterogeneously catalysed hydrogenation using gold catalysts. Appl. Catal. A Gen. 2005, 291, 222–229. [Google Scholar] [CrossRef]
- Becker, M.J. Etruscan gold dental appliances. In Molecular and Structural Archaeology: Cosmetic and Therapeutic Chemicals; Springer: Berlin, Germany, 2003; pp. 11–27. [Google Scholar]
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.; El-Sayed, I.H.; Chu, H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008, 269, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, S.; Schwalb, W.; Wang, Y.; Chen, Y.; Tang, Y.; Si, J.; Shirinzadeh, B.; Cheng, W. A wearable and highly sensitive pressure sensor with ultrathin gold nanowires. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, L.; Jackson, J.; Lee, A.; Halas, N.; West, J. A whole blood immunoassay using gold nanoshells. Anal. Chem. 2003, 75, 2377–2381. [Google Scholar] [CrossRef]
- Herzing, A.A.; Kiely, C.J.; Carley, A.F.; Landon, P.; Hutchings, G.J. Identification of active gold nanoclusters on iron oxide supports for CO oxidation. Science 2008, 321, 1331–1335. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, C.; Xu, Z.; Lin, H.; Zhang, C. Gold nanoparticle-enhanced near infrared fluorescent nanocomposites for targeted bio-imaging. RSC Adv. 2015, 5, 20–26. [Google Scholar] [CrossRef]
- Aydogan, B.; Li, J.; Rajh, T.; Chaudhary, A.; Chmura, S.J.; Pelizzari, C.; Wietholt, C.; Kurtoglu, M.; Redmond, P. AuNP-DG: Deoxyglucose-labeled gold nanoparticles as X-ray computed tomography contrast agents for cancer imaging. Mol. Imaging Boil. 2010, 12, 463–467. [Google Scholar] [CrossRef]
- Vieira, L.; Castilho, M.; Ferreira, I.; Ferreira-Strixino, J.; Hewitt, K.; Raniero, L. Synthesis and characterization of gold nanostructured Chorin e6 for Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2017, 18, 6–11. [Google Scholar] [CrossRef]
- Hwang, S.; Nam, J.; Jung, S.; Song, J.; Doh, H.; Kim, S. Gold nanoparticle-mediated photothermal therapy: Current status and future perspective. Nanomedicine 2014, 9, 2003–2022. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, H.; Tang, S.; Li, Y.; Yu, X.-F.; Wang, H.; Li, P.; Sun, Z.; Zhang, H.; Liu, C. Small gold nanorods laden macrophages for enhanced tumor coverage in photothermal therapy. Biomaterials 2016, 74, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Popp, M.K.; Oubou, I.; Shepherd, C.; Nager, Z.; Anderson, C.; Pagliaro, L. Photothermal therapy using gold nanorods and near-infrared light in a murine melanoma model increases survival and decreases tumor volume. J. Nanomater. 2014, 2014, 8. [Google Scholar] [CrossRef]
- Du, C.; Wang, A.; Fei, J.; Zhao, J.; Li, J. Polypyrrole-stabilized gold nanorods with enhanced photothermal effect towards two-photon photothermal therapy. J. Mater. Chem. B 2015, 3, 4539–4545. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Matsuki, D.; Okajima, J.; Komiya, A.; Mori, S.; Maruyama, S.; Kodama, T. Photothermal therapy of tumors in lymph nodes using gold nanorods and near-infrared laser light with controlled surface cooling. Nano Res. 2015, 8, 3842–3852. [Google Scholar] [CrossRef]
- Carpin, L.B.; Bickford, L.R.; Agollah, G.; Yu, T.-K.; Schiff, R.; Li, Y.; Drezek, R.A. Immunoconjugated gold nanoshell-mediated photothermal ablation of trastuzumab-resistant breast cancer cells. Breast Cancer Res. Treat. 2011, 125, 27–34. [Google Scholar] [CrossRef]
- Kennedy, L.C. Modulating Gold Nanoparticle In Vivo Delivery for Photothermal Therapy Applications Using a T Cell Delivery System. Ph.D. Thesis, Rice University, Houston, TX, USA, 2012. [Google Scholar]
- Huang, J.; Guo, M.; Ke, H.; Zong, C.; Ren, B.; Liu, G.; Shen, H.; Ma, Y.; Wang, X.; Zhang, H. Rational design and synthesis of γFe2O3@ Au magnetic gold nanoflowers for efficient cancer theranostics. Adv. Mater. 2015, 27, 5049–5056. [Google Scholar] [CrossRef]
- Han, J.; Li, J.; Jia, W.; Yao, L.; Li, X.; Jiang, L.; Tian, Y. Photothermal therapy of cancer cells using novel hollow gold nanoflowers. Int. J. Nanomed. 2014, 9, 517–526. [Google Scholar]
- Chu, C.-K.; Tu, Y.-C.; Chang, Y.-W.; Chu, C.-K.; Chen, S.-Y.; Chi, T.-T.; Kiang, Y.-W.; Yang, C.-C. Cancer cell uptake behavior of Au nanoring and its localized surface plasmon resonance induced cell inactivation. Nanotechnology 2015, 26, 075102. [Google Scholar] [CrossRef]
- Camerin, M.; Magaraggia, M.; Soncin, M.; Jori, G.; Moreno, M.; Chambrier, I.; Cook, M.J.; Russell, D.A. The in vivo efficacy of phthalocyanine-nanoparticle conjugates for the photodynamic therapy of amelanotic melanoma. Eur. J. Cancer 2010, 46, 1910–1918. [Google Scholar] [CrossRef]
- Stuchinskaya, T.; Moreno, M.; Cook, M.J.; Edwards, D.R.; Russell, D.A. Targeted photodynamic therapy of breast cancer cells using antibody-phthalocyanine-gold nanoparticle conjugates. Photochem. Photobiol. Sci. 2011, 10, 822–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savarimuthu, W.P.; Gananathan, P.; Rao, A.P.; Manickam, E.; Singaravelu, G. Protoporphyrin IX-gold nanoparticle conjugates for targeted photodynamic therapy-an In-vitro study. J. Nanosci. Nanotechnol. 2015, 15, 5577–5584. [Google Scholar] [CrossRef] [PubMed]
- Gamaleia, N.; Shishko, E.; Dolinsky, G.; Shcherbakov, A.; Usatenko, A.; Kholin, V. Photodynamic activity of hematoporphyrin conjugates with gold nanoparticles: Experiments in vitro. Exp. Oncol. 2010, 32, 44–47. [Google Scholar] [PubMed]
- Khaing Oo, M.K.; Yang, Y.; Hu, Y.; Gomez, M.; Du, H.; Wang, H. Gold nanoparticle-enhanced and size-dependent generation of reactive oxygen species from protoporphyrin IX. ACS Nano 2012, 6, 1939–1947. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Xu, H.; Wang, B.; Yao, C. Role of 5-aminolevulinic acid-conjugated gold nanoparticles for photodynamic therapy of cancer. J. Biomed. Opt. 2015, 20, 051043. [Google Scholar] [CrossRef]
- Kuo, W.S.; Chang, C.N.; Chang, Y.T.; Yang, M.H.; Chien, Y.H.; Chen, S.J.; Yeh, C.S. Gold nanorods in photodynamic therapy, as hyperthermia agents, and in near-infrared optical imaging. Angew. Chem. Int. Ed. 2010, 49, 2711–2715. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Fei, J.; Zhao, J.; Li, H.; Cui, Y.; Li, J. Hypocrellin-loaded gold nanocages with high two-photon efficiency for photothermal/photodynamic cancer therapy in vitro. ACS Nano 2012, 6, 8030–8040. [Google Scholar] [CrossRef]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G. Single continuous wave laser induced photodynamic/plasmonic photothermal therapy using photosensitizer-functionalized gold nanostars. Adv. Mater. 2013, 25, 3055–3061. [Google Scholar] [CrossRef] [Green Version]
- Kuo, W.-S.; Chang, Y.-T.; Cho, K.-C.; Chiu, K.-C.; Lien, C.-H.; Yeh, C.-S.; Chen, S.-J. Gold nanomaterials conjugated with indocyanine green for dual-modality photodynamic and photothermal therapy. Biomaterials 2012, 33, 3270–3278. [Google Scholar] [CrossRef]
- Jang, B.; Park, J.-Y.; Tung, C.-H.; Kim, I.-H.; Choi, Y. Gold nanorod—Photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano 2011, 5, 1086–1094. [Google Scholar] [CrossRef]
- Terentyuk, G.; Panfilova, E.; Khanadeev, V.; Chumakov, D.; Genina, E.; Bashkatov, A.; Tuchin, V.; Bucharskaya, A.; Maslyakova, G.; Khlebtsov, N. Gold nanorods with a hematoporphyrin-loaded silica shell for dual-modality photodynamic and photothermal treatment of tumors in vivo. Nano Res. 2014, 7, 325–337. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, X.; Tang, P.; Zhang, D.; Tian, J.; Zhong, L. Gold nanoparticle (AuNP)-based surface-enhanced Raman scattering (SERS) probe of leukemic lymphocytes. Plasmonics 2016, 11, 1361–1368. [Google Scholar] [CrossRef]
- Tam, N.C.; McVeigh, P.Z.; MacDonald, T.D.; Farhadi, A.; Wilson, B.C.; Zheng, G. Porphyrin-lipid stabilized gold nanoparticles for surface enhanced raman scattering based imaging. Bioconj. Chem. 2012, 23, 1726–1730. [Google Scholar] [CrossRef]
- Quynh, L.M.; Nam, N.H.; Kong, K.; Nhung, N.T.; Notingher, I.; Henini, M.; Luong, N.H. Surface-enhanced Raman spectroscopy study of 4-ATP on gold nanoparticles for basal cell carcinoma fingerprint detection. J. Electron. Mater. 2016, 45, 2563–2568. [Google Scholar] [CrossRef]
- Lai, S.-F.; Chien, C.-C.; Chen, W.-C.; Chen, H.-H.; Chen, Y.-Y.; Wang, C.-L.; Hwu, Y.; Yang, C.; Chen, C.; Liang, K. Very small photoluminescent gold nanoparticles for multimodality biomedical imaging. Biotechnol. Adv. 2013, 31, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Suarasan, S.; Licarete, E.; Astilean, S.; Craciun, A.-M. Probing cellular uptake and tracking of differently shaped gelatin-coated gold nanoparticles inside of ovarian cancer cells by two-photon excited photoluminescence analyzed by fluorescence lifetime imaging (FLIM). Colloids Surf. B Biointerfaces 2018, 166, 135–143. [Google Scholar] [CrossRef]
- Patskovsky, S.; Bergeron, E.; Meunier, M. Hyperspectral darkfield microscopy of PEGylated gold nanoparticles targeting CD44-expressing cancer cells. J. Biophotonics 2013, 8, 162–167. [Google Scholar] [CrossRef]
- Gong, T.; Olivo, M.; Dinish, U.; Goh, D.; Kong, K.V.; Yong, K.-T. Engineering bioconjugated gold nanospheres and gold nanorods as label-free plasmon scattering probes for ultrasensitive multiplex dark-field imaging of cancer cells. J. Biomed. Nanotechnol. 2013, 9, 985–991. [Google Scholar] [CrossRef]
- Hu, J.; Sanz-Rodríguez, F.; Rivero, F.; Rodríguez, E.M.; Torres, R.A.; Ortgies, D.H.; Solé, J.G.; Alfonso, F.; Jaque, D. Gold nanoshells: Contrast agents for cell imaging by cardiovascular optical coherence tomography. Nano Res. 2018, 11, 676–685. [Google Scholar] [CrossRef]
- Xiao, P.; Li, Q.; Joo, Y.; Nam, J.; Hwang, S.; Song, J.; Kim, S.; Joo, C.; Kim, K.H. Detection of pH-induced aggregation of “smart” gold nanoparticles with photothermal optical coherence tomography. Opt. Lett. 2013, 38, 4429–4432. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Bui, N.Q.; Jang, B.; Oh, Y.-O.; Lim, I.G.; Oh, J. Doxorubicin-loaded fucoidan capped gold nanoparticles for drug delivery and photoacoustic imaging. Int. J. Boil. Macromol. 2016, 91, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, J.; Yang, K.; Yi, C.; Liu, Y.; Nie, L.; Khashab, N.M.; Chen, X.; Nie, Z. Folding up of gold nanoparticle strings into plasmonic vesicles for enhanced photoacoustic imaging. Angew. Chem. Int. Ed. 2015, 54, 15809–15812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallidi, S.; Larson, T.; Tam, J.; Joshi, P.P.; Karpiouk, A.; Sokolov, K.; Emelianov, S. Multiwavelength photoacoustic imaging and plasmon resonance coupling of gold nanoparticles for selective detection of cancer. Nano Lett. 2009, 9, 2825–2831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albanese, A.; Chan, W.C. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano 2011, 5, 5478–5489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-D.; di Wu, X.S.; Liu, P.-X.; Yang, N.; Zhao, B.; Zhang, H.; Sun, Y.-M.; Zhang, L.-A.; Fan, F.-Y. Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int. J. Nanomed. 2011, 6, 2071–2081. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Lu, W.; Tovmachenko, O.; Rai, U.S.; Yu, H.; Ray, P.C. Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem. Phys. Lett. 2008, 463, 145–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef]
- Feliu, N.; Docter, D.; Heine, M.; del Pino, P.; Ashraf, S.; Kolosnjaj-Tabi, J.; Macchiarini, P.; Nielsen, P.; Alloyeau, D.; Gazeau, F. In vivo degeneration and the fate of inorganic nanoparticles. Chem. Soc. Rev. 2016, 45, 2440–2457. [Google Scholar] [CrossRef] [Green Version]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef]
- Murphy, C.J.; Gole, A.M.; Stone, J.W.; Sisco, P.N.; Alkilany, A.M.; Goldsmith, E.C.; Baxter, S.C. Gold nanoparticles in biology: Beyond toxicity to cellular imaging. Acc. Chem. Res. 2008, 41, 1721–1730. [Google Scholar] [CrossRef]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Size, shape and surface chemistry of nano-gold dictate its cellular interactions, uptake and toxicity. Prog. Mater. Sci. 2016, 83, 152–190. [Google Scholar] [CrossRef]
- Kobayashi, K.; Wei, J.; Iida, R.; Ijiro, K.; Niikura, K. Surface engineering of nanoparticles for therapeutic applications. Polym. J. 2014, 46, 460–468. [Google Scholar] [CrossRef]
- Zhu, Y.; Chandra, P.; Song, K.-M.; Ban, C.; Shim, Y.-B. Label-free detection of kanamycin based on the aptamer-functionalized conducting polymer/gold nanocomposite. Biosens. Bioelectron. 2012, 36, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Duy, J.; Connell, L.B.; Eck, W.; Collins, S.D.; Smith, R.L. Preparation of surfactant-stabilized gold nanoparticle–peptide nucleic acid conjugates. J. Nanopart. Res. 2010, 12, 2363–2369. [Google Scholar] [CrossRef]
- Charbgoo, F.; Nejabat, M.; Abnous, K.; Soltani, F.; Taghdisi, S.M.; Alibolandi, M.; Shier, W.T.; Steele, T.W.; Ramezani, M. Gold nanoparticle should understand protein corona for being a clinical nanomaterial. J. Control. Release 2018, 272, 39–53. [Google Scholar] [CrossRef]
- Conde, J.; Dias, J.T.; Grazú, V.; Moros, M.; Baptista, P.V.; de la Fuente, J.M. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014, 2, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, A.; Zeng, C.; Zhou, M.; Jin, R. Surface engineering of Au36 (SR) 24 nanoclusters for photoluminescence enhancement. Part. Part. Syst. Charact. 2017, 34, 1600388. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Huang, H.; Liu, G.; Zhou, W.; Chen, Y.; Jin, Q.; Ji, J. Multidentate zwitterionic chitosan oligosaccharide modified gold nanoparticles: Stability, biocompatibility and cell interactions. Nanoscale 2013, 5, 3982. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.; Park, J.-H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51. [Google Scholar]
- Vácha, R.; Martinez-Veracoechea, F.J.; Frenkel, D. Receptor-mediated endocytosis of nanoparticles of various shapes. Nano Lett. 2011, 11, 5391–5395. [Google Scholar] [CrossRef]
- Vecchio, G.; Galeone, A.; Brunetti, V.; Maiorano, G.; Sabella, S.; Cingolani, R.; Pompa, P.P. Concentration-dependent, size-independent toxicity of citrate capped AuNPs in Drosophila melanogaster. PLoS ONE 2012, 7, e29980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangabathuni, S.; Murthy, R.V.; Chaudhary, P.M.; Subramani, B.; Toraskar, S.; Kikkeri, R. Mapping the glyco-gold nanoparticles of different shapes toxicity, biodistribution and sequestration in adult zebrafish. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.K. Determinants of tumor blood flow: A review. Cancer Res. 1988, 48, 2641–2658. [Google Scholar] [PubMed]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R.K. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar] [PubMed]
- Shukla, T.; Upmanyu, N.; Pandey, S.P.; Sudheesh, M. Site-specific drug delivery, targeting, and gene therapy. In Nanoarchitectonics in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 473–505. [Google Scholar]
- Ahangari, A.; Salouti, M.; Saghatchi, F. Gentamicin-gold nanoparticles conjugate: A contrast agent for X-ray imaging of infectious foci due to staphylococcus aureus. IET Nanobiotechnol. 2016, 10, 190–194. [Google Scholar] [CrossRef]
- Xing, S.; Zhang, X.; Luo, L.; Cao, W.; Li, L.; He, Y.; An, J.; Gao, D. Doxorubicin/gold nanoparticles coated with liposomes for chemo-photothermal synergetic antitumor therapy. Nanotechnology 2018, 29, 405101. [Google Scholar] [CrossRef]
- Chauhan, G.; Chopra, V.; Tyagi, A.; Rath, G.; Sharma, R.K.; Goyal, A.K. “Gold nanoparticles composite-folic acid conjugated graphene oxide nanohybrids” for targeted chemo-thermal cancer ablation: In vitro screening and in vivo studies. Eur. J. Pharm. Sci. 2017, 96, 351–361. [Google Scholar] [CrossRef]
- Cole, L.E.; Ross, R.D.; Tilley, J.M.; Vargo-Gogola, T.; Roeder, R.K. Gold nanoparticles as contrast agents in x-ray imaging and computed tomography. Nanomedicine 2015, 10, 321–341. [Google Scholar] [CrossRef] [Green Version]
- Chien, C.-C.; Chen, H.-H.; Lai, S.-F.; Wu, K.-C.; Cai, X.; Hwu, Y.; Petibois, C.; Chu, Y.; Margaritondo, G. Gold nanoparticles as high-resolution X-ray imaging contrast agents for the analysis of tumor-related micro-vasculature. J. Nanobiotechnol. 2012, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Iwakuma, N.; Sharma, P.; Moudgil, B.; Wu, C.; McNeill, J.; Jiang, H.; Grobmyer, S. Gold nanoparticles as a contrast agent for in vivo tumor imaging with photoacoustic tomography. Nanotechnology 2009, 20, 395102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lee, K.; Kim, I.K.; Park, T.G. Synthesis, characterization, and in vivo diagnostic applications of hyaluronic acid immobilized gold nanoprobes. Biomaterials 2008, 29, 4709–4718. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ali, M.R.; Chen, K.; Fang, N.; El-Sayed, M.A. Gold nanoparticles in biological optical imaging. Nano Today 2019, 24, 120–140. [Google Scholar] [CrossRef]
- Qu, Y.; Liu, F.; Wei, Y.; Gu, C.; Zhang, L.; Liu, Y. Forming ceria shell on Au-core by LSPR photothermal induced interface reaction. Appl. Surf. Sci. 2015, 343, 207–211. [Google Scholar] [CrossRef]
- Kim, S.-E.; Lee, B.-R.; Lee, H.; Jo, S.D.; Kim, H.; Won, Y.-Y.; Lee, J. Near-infrared plasmonic assemblies of gold nanoparticles with multimodal function for targeted cancer theragnosis. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; van Duyne, R.P. Biosensing with plasmonic nanosensors, Nanoscience and Technology: A Collection of Reviews from Nature Journals. World Scientific: Singapore, Singapore, 2010; pp. 308–319. [Google Scholar]
- Chou, C.-H.; Chen, C.-D.; Wang, C.C. Highly efficient, wavelength-tunable, gold nanoparticle based optothermal nanoconvertors. J. Phys. Chem. B 2005, 109, 11135–11138. [Google Scholar] [CrossRef]
- Jo, W.; Freedman, K.; Yi, D.K.; Bose, R.K.; Lau, K.K.; Solomon, S.D.; Kim, M.J. Photon to thermal response of a single patterned gold nanorod cluster under near-infrared laser irradiation. Biofabrication 2011, 3, 015002. [Google Scholar] [CrossRef]
- Meyer, S.A.; Ru, E.C.L.; Etchegoin, P.G. Quantifying resonant Raman cross sections with SERS. J. Phys. Chem. A 2010, 114, 5515–5519. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667. [Google Scholar] [CrossRef] [Green Version]
- Kneipp, K.; Kneipp, H.; Manoharan, R.; Hanlon, E.B.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Extremely large enhancement factors in surface-enhanced Raman scattering for molecules on colloidal gold clusters. Appl. Spectrosc. 1998, 52, 1493–1497. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Kartha, V.B.; Manoharan, R.; Deinum, G.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Detection and identification of a single DNA base molecule using surface-enhanced Raman scattering (SERS). Phys. Rev. E 1998, 57, R6281. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Nguyen, J.T.; Rutledge, S.; Zhang, J.; Wang, C.; Walker, G.C. Detection of chronic lymphocytic leukemia cell surface markers using surface enhanced Raman scattering gold nanoparticles. Cancer Lett. 2010, 292, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Lin, J.; Cheng, M.; Li, Y.-Z.; Chen, G.; Huang, Z.; Yu, Y.; Chen, R.; Zeng, H. Gold nanoparticle based surface-enhanced Raman scattering spectroscopy of cancerous and normal nasopharyngeal tissues under near-infrared laser excitation. Appl. Spectrosc. 2009, 63, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Tuan, V.-D.; Allain, L.R.; Stokes, D.L. Cancer gene detection using surface-enhanced Raman scattering (SERS). J. Raman Spectrosc. 2002, 33, 511–516. [Google Scholar]
- O’Neal, D.P.; Hirsch, L.R.; Halas, N.J.; Payne, J.D.; West, J.L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004, 209, 171–176. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Huang, X.; Qian, W.; El-Sayed, I.H.; El-Sayed, M.A. The potential use of the enhanced nonlinear properties of gold nanospheres in photothermal cancer therapy. Lasers Surg. Med.: Off. J. Am. Soc. Laser Med. Surg. 2007, 39, 747–753. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef]
- Lapotko, D.; Lukianova, E.; Potapnev, M.; Aleinikova, O.; Oraevsky, A. Method of laser activated nano-thermolysis for elimination of tumor cells. Cancer Lett. 2006, 239, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yao, C.; Wang, J.; Li, Z.; Zhang, Z. Anti-CD30-targeted gold nanoparticles for photothermal therapy of L-428 Hodgkin’s cell. Int. J. Nanomed. 2012, 7, 6095–6103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, K.C.; Yi, J.; Rivera, J.G.; Zelasko-Leon, D.C.; Messersmith, P.B. Polydopamine-enabled surface functionalization of gold nanorods for cancer cell-targeted imaging and photothermal therapy. Nanomedicine 2013, 8, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkilany, A.M.; Thompson, L.B.; Boulos, S.P.; Sisco, P.N.; Murphy, C.J. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv. Drug Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Dembereldorj, U.; Choi, S.Y.; Ganbold, E.-O.; Song, N.W.; Kim, D.; Choo, J.; Lee, S.Y. Gold nanorod-assembled PEGylated graphene-oxide nanocomposites for photothermal cancer therapy. Photochem. Photobiol. 2014, 90, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, G.; You, M.; Song, E.; Shukoor, M.I.; Zhang, K.; Altman, M.B.; Chen, Y.; Zhu, Z.; Huang, C.Z. Assembly of aptamer switch probes and photosensitizer on gold nanorods for targeted photothermal and photodynamic cancer therapy. ACS Nano 2012, 6, 5070–5077. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Liu, X.; Hu, T.; Chu, P.K. Ultra-sensitive detection of cysteine by gold nanorod assembly. Biosens. Bioelectron. 2010, 25, 2078–2083. [Google Scholar] [CrossRef]
- Seo, S.-H.; Kim, B.-M.; Joe, A.; Han, H.-W.; Chen, X.; Cheng, Z.; Jang, E.-S. NIR-light-induced surface-enhanced Raman scattering for detection and photothermal/photodynamic therapy of cancer cells using methylene blue-embedded gold nanorod@ SiO2 nanocomposites. Biomaterials 2014, 35, 3309–3318. [Google Scholar] [CrossRef] [Green Version]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv. Mater. 2001, 13, 1389–1393. [Google Scholar] [CrossRef]
- Vankayala, R.; Lin, C.-C.; Kalluru, P.; Chiang, C.-S.; Hwang, K.C. Gold nanoshells-mediated bimodal photodynamic and photothermal cancer treatment using ultra-low doses of near infra-red light. Biomaterials 2014, 35, 5527–5538. [Google Scholar] [CrossRef]
- Pham, T.; Jackson, J.B.; Halas, N.J.; Lee, T.R. Preparation and characterization of gold nanoshells coated with self-assembled monolayers. Langmuir 2002, 18, 4915–4920. [Google Scholar] [CrossRef]
- Trinidad, A.J.; Hong, S.J.; Peng, Q.; Madsen, S.J.; Hirschberg, H. Combined concurrent photodynamic and gold nanoshell loaded macrophage?mediated photothermal therapies: An in vitro study on squamous cell head and neck carcinoma. Lasers Surg. Med. 2014, 46, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, F.; Spasova, M.; Salgueiri-o-Maceira, V.; Liz-Marz-n, L. Multilayer assemblies of silica-encapsulated gold nanoparticles on decomposable colloid templates. Adv. Mater. 2001, 13, 1090–1094. [Google Scholar] [CrossRef]
- Oldenburg, S.; Averitt, R.; Westcott, S.; Halas, N. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Chhetri, S.; Hirschberg, H.; Madsen, S.J. Photothermal therapy of human glioma spheroids with gold-silica nanoshells and gold nanorods: A comparative study. In Proceedings of Optical Techniques in Neurosurgery, Neurophotonics, and Optogenetics; SPIE: Bellingham, WA, USA, 2014; p. 89280U. [Google Scholar]
- Kang, S.; Bhang, S.H.; Hwang, S.; Yoon, J.-K.; Song, J.; Jang, H.-K.; Kim, S.; Kim, B.-S. Mesenchymal stem cells aggregate and deliver gold nanoparticles to tumors for photothermal therapy. ACS Nano 2015, 9, 9678–9690. [Google Scholar] [CrossRef]
- Tada, D.B.; Baptista, M.S. Photosensitizing nanoparticles and the modulation of ROS generation. Front. Chem. 2015, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H. Type I and type II photosensitized oxidation reactions: Guidelines and mechanistic pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef] [Green Version]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Yong, Z. Upconverting nanoparticles as nanotransducers for photodynamic therapy in cancer cells. Nanomedicine 2008, 3, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Hodak, J.H.; Henglein, A.; Hartland, G.V. Photophysics of nanometer sized metal particles: Electron-Phonon coupling and coherent excitation of breathing vibrational modes. J. Phys. Chem. B 2000, 104, 9954–9965. [Google Scholar] [CrossRef]
- Jia, X.; Jia, L. Nanoparticles improve biological functions of phthalocyanine photosensitizers used for photodynamic therapy. Curr. Drug Metab. 2012, 13, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Samia, A.C.; Meyers, J.D.; Panagopoulos, I.; Fei, B.; Burda, C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J. Am. Chem. Soc. 2008, 130, 10643–10647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Chen, Z.; Zhao, H.; Zha, Z.; Ke, W.; Wang, Y.; Ge, Z. Oxygen-independent combined photothermal/photodynamic therapy delivered by tumor acidity-responsive polymeric micelles. J. Control. Release 2018, 284, 15–25. [Google Scholar]
- Hone, D.C.; Walker, P.I.; Evans-Gowing, R.; FitzGerald, S.; Beeby, A.; Chambrier, I.; Cook, M.J.; Russell, D.A. Generation of cytotoxic singlet oxygen via phthalocyanine-stabilized gold nanoparticles: A potential delivery vehicle for photodynamic therapy. Langmuir 2002, 18, 2985–2987. [Google Scholar] [CrossRef]
- Wieder, M.E.; Hone, D.C.; Cook, M.J.; Handsley, M.M.; Gavrilovic, J.; Russell, D.A. Intracellular photodynamic therapy with photosensitizer-nanoparticle conjugates: Cancer therapy using a ‘Trojan horse’. Photochem. Photobiol. Sci. 2006, 5, 727–734. [Google Scholar] [CrossRef]
- Khaing Oo, M.K.; Yang, X.; Du, H.; Wang, H. 5-aminolevulinic acid-conjugated gold nanoparticles for photodynamic therapy of cancer. Nanomedicine 2008, 3, 777–786. [Google Scholar] [CrossRef]
- Vendrell, M.; Maiti, K.K.; Dhaliwal, K.; Chang, Y.-T. Surface-enhanced Raman scattering in cancer detection and imaging. Trends Biotechnol. 2013, 31, 249–257. [Google Scholar] [CrossRef]
- Farrer, R.A.; Butterfield, F.L.; Chen, V.W.; Fourkas, J.T. Highly efficient multiphoton-absorption-induced luminescence from gold nanoparticles. Nano Lett. 2005, 5, 1139–1142. [Google Scholar] [CrossRef]
- Nagesha, D.; Laevsky, G.; Lampton, P.; Banyal, R.; Warner, C.; DiMarzio, C.; Sridhar, S. In vitro imaging of embryonic stem cells using multiphoton luminescence of gold nanoparticles. Int. J. Nanomed. 2007, 2, 813. [Google Scholar]
- Li, T.; Wu, X.; Liu, F.; Li, N. Analytical methods based on the light-scattering of plasmonic nanoparticles at the single particle level with dark-field microscopy imaging. Analyst 2017, 142, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Braz, A.K.S.; de Araujo, R.E.; Ohulchanskyy, T.Y.; Shukla, S.; Bergey, E.J.; Gomes, A.S.; Prasad, P.N. In situ gold nanoparticles formation: Contrast agent for dental optical coherence tomography. J. Biomed. Opt. 2012, 17, 066003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luke, G.P.; Yeager, D.; Emelianov, S.Y. Biomedical applications of photoacoustic imaging with exogenous contrast agents. Ann. Biomed. Eng. 2012, 40, 422–437. [Google Scholar] [CrossRef]






| Classification | Theragnostic Modules | Required Characteristics | NP Formulation | NP Modification | Experimental Methods | Reference |
|---|---|---|---|---|---|---|
| Phototherapy | PTT | LSPR | Rod | BSA, Macrophage | In vivo | [14] |
| LSPR | Rod | None | In vitro and In vivo | [15] | ||
| TPA | Rod | Polypyrrole coating | In vitro | [16] | ||
| LSPR/EPR effect | Rod | None | In vivo | [17] | ||
| LSPR | Shell | Anti-HER2 antibody, silica | In vitro | [18] | ||
| LSPR | Shell | T cell | In vivo | [19] | ||
| LSPR | Flowers | γFe2o2 | In vivo | [20] | ||
| LSPR | Flowers | None | In vitro and In vivo | [21] | ||
| LSPR | Ring | Antiibody | In vitro | [22] | ||
| PDT | EPR effect | Sphere | C11Pc | In vitro and In vivo | [23] | |
| EPR effect | Sphere | Antibody, C11Pc, PEG | In vitro and In vivo | [24] | ||
| EPR effect | Sphere | Protoporphyrin IX | In vitro | [25] | ||
| EPR effect | Sphere | Hematoporphyrin | In vitro | [26] | ||
| LSPR | Sphere | Protoporphyrin IX | In vitro | [27] | ||
| EPR effect | Sphere | 5-aminolevulinic acid | In vitro | [28] | ||
| LSPR/EPR effect | Rod | Indocyanine green | In vitro | [29] | ||
| PTT & PDT | LSPR | Cages | Hypocrellin | In vitro | [30] | |
| LSPR | Stars | Chlorin e6 | In vivo | [31] | ||
| LSPR/EPR effect | Rod | Indocyanine green | In vitro | [32] | ||
| LSPR/EPR effect | Rod | Cetyltrimethylammonium bromide, PEG | In vivo | [33] | ||
| LSPR | Rod | Hematoporphyrin, Silica | In vivo | [34] | ||
| Imaging | SERS | LSPR/SERS | Sphere | Anti-CD3/CD19 antibody, MBA, PEG | In vitro | [35] |
| LSPR/SERS | Sphere | Mn, porphyrin-phospholipid | In vitro | [36] | ||
| LSPR/SERS | Sphere | 4-ATP attachment | ex vivo | [37] | ||
| Multiphoton photoluminescence | Photoluminescence | Sphere | MUA | In vitro and In vivo | [38] | |
| Photoluminescence | Sphere, rod, triangle | Gelatin coating | In vitro | [39] | ||
| Dark field microscopy | LSPR | Sphere | Anti-CD44 antibody, PEG | In vitro | [40] | |
| LSPR | Sphere, rod | Anti-EGFR antibody, PEG | In vitro | [41] | ||
| OCT | Absorption property, LSPR | Shell | None | In vivo | [42] | |
| Photothermal property, absorption property, LSPR | Sphere | Hydrolysis-susceptible Citraconic amide | In vitro | [43] | ||
| PAI | LSPR, PA | Sphere | DOX loading, Fu capping | In vitro | [44] | |
| LSPR, PA | Sphere string | BCP tethering | In vivo | [45] | ||
| LSPR, PA | Sphere | Anti-EGFR antibody | ex vivo | [46] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.-W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics 2020, 12, 701. https://doi.org/10.3390/pharmaceutics12080701
Kang MS, Lee SY, Kim KS, Han D-W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics. 2020; 12(8):701. https://doi.org/10.3390/pharmaceutics12080701
Chicago/Turabian StyleKang, Moon Sung, So Yun Lee, Ki Su Kim, and Dong-Wook Han. 2020. "State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis" Pharmaceutics 12, no. 8: 701. https://doi.org/10.3390/pharmaceutics12080701
APA StyleKang, M. S., Lee, S. Y., Kim, K. S., & Han, D. -W. (2020). State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics, 12(8), 701. https://doi.org/10.3390/pharmaceutics12080701