Micelles of Progesterone for Topical Eye Administration: Interspecies and Intertissues Differences in Ex Vivo Ocular Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Micelles Preparation and Characterization
2.3. Solubility of Progesterone (PG) in Micelle Dispersions
2.4. Rheological Analysis
2.5. Hen’s Egg Test Chorioallantoic Membrane (HET-CAM)
2.6. Ex Vivo Corneal and Sclera Permeability Assay
2.7. Statistical Analysis
3. Results and Discussion
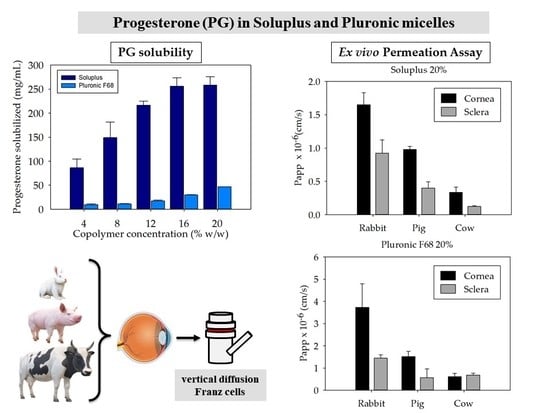
3.1. Micelles Preparation and PG Solubilization
3.2. Rheological Properties of the Formulations
3.3. HET-CAM Test
3.4. Ex Vivo Permeation Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Y.J.; Peng, J.; Ying, D.; Peng, Q.H. A brief review on the pathological role of decreased blood flow affected in retinitis pigmentosa. J. Ophthalmol. 2018, 2018, 3249064. [Google Scholar] [CrossRef] [Green Version]
- Jauregui, R.; Park, K.S.; Duong, J.K.; Mahajan, V.B.; Tsang, S.H. Quantitative progression of retinitis pigmentosa by optical coherence tomography angiography. Sci. Rep. 2018, 8, 13130. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Sahni, J.N.; Angi, M.; Irigoyen, C.; Semeraro, F.; Romano, M.R.; Parmeggiani, F. Therapeutic challenges to retinitis pigmentosa: From neuroprotection to gene therapy. Curr. Genom. 2011, 12, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komeima, K.; Rogers, B.S.; Campochiaro, P.A. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell Physiol. 2007, 213, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, R.; Scalabrin, S.; Becco, A.; Panzica, G. Sex hormones and optic nerve disorders: A review. Front. Neurosci. 2019, 13, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz Lopez, A.M.; Roche, S.L.; Wyse Jackson, A.C.; Moloney, J.N.; Byrne, A.M.; Cotter, T.G. Pro-survival redox signalling in progesterone-mediated retinal neuroprotection. Eur. J. Neurosci. 2017, 46, 1663–1672. [Google Scholar] [CrossRef]
- Doonan, F.; O’Driscoll, C.; Kenna, P.; Cotter, T.G. Enhancing survival of photoreceptor cells in vivo using the synthetic progestin Norgestrel. J. Neurochem. 2011, 118, 915–927. [Google Scholar] [CrossRef]
- Hernández-Rabaza, V.; López-Pedrajas, R.; Almansa, I. Progesterone, lipoic acid, and sulforaphane as promising antioxidants for retinal diseases: A review. Antioxidants 2019, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Vallejo, V.; Benlloch-Navarro, S.; López-Pedrajas, R.; Romero, F.J.; Miranda, M. Neuroprotective actions of progesterone in an in vivo model of retinitis pigmentosa. Pharmacol. Res. 2015, 99, 276–288. [Google Scholar] [CrossRef]
- Nuzzi, R.; Scalabrin, S.; Becco, A.; Panzica, G. Gonadal hormones and retinal disorders: A review. Front. Endocrinol. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, R.S.; Olsen, T.W.; Sayeed, I.; Cale, H.A.; Morrison, K.C.; Oumarbaeva, Y.; Lucaciu, I.; Boatright, J.H.; Pardue, M.T.; Stein, D.G. Progesterone treatment in two rat models of ocular ischemia. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2880–2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, A.S.; Soliman, G.M.; El-Mahdy, M.M.; El-Gindy, G.E.A. Solubilization and enhancement of ex vivo vaginal delivery of progesterone using solid dispersions, inclusion complexes and micellar solubilization. Curr. Drug Deliv. 2017, 15, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Li, V.H.K.; Wood, R.W.; Kreuter, J.; Harmia, T.; Robinson, J.R. Ocular drug delivery of progesterone using nanoparticles. J. Microencapsul. 1986, 3, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Vadlapudi, A.D.; Cholkar, K.; Vadlapatla, R.K.; Mitra, A.K. Aqueous nano micellar formulation for topical delivery of biotinylated lipid prodrug of acyclovir: Formulation development and ocular biocompatibility. J. Ocul. Pharmacol. Ther. 2014, 30, 49–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barar, J.; Aghanejad, A.; Fathi, M.; Omidi, Y. Advanced drug delivery and targeting technologies for the ocular diseases. BioImpacts 2016, 6, 49–67. [Google Scholar] [CrossRef] [Green Version]
- Kang-Mieler, J.J.; Dosmar, E.; Liu, W.; Mieler, W.F. Extended ocular drug delivery systems for the anterior and posterior segments: Biomaterial options and applications. Expert Opin. Drug Deliv. 2017, 14, 611–620. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular drug delivery: Present innovations and future challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Soliman, O.A.E.-A.; Mohamed, E.A.; Khatera, N.A.A. Enhanced ocular bioavailability of fluconazole from niosomal gels and microemulsions: Formulation, optimization, and in vitro–in vivo evaluation. Pharm. Dev. Technol. 2019, 24, 48–62. [Google Scholar] [CrossRef]
- Bachu, R.; Chowdhury, P.; Al-Saedi, Z.; Karla, P.; Boddu, S. Ocular drug delivery barriers—Role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, E.; del Amo, E.M.; Toropainen, E.; Tengvall-Unadike, U.; Ranta, V.P.; Urtti, A.; Ruponen, M. Corneal and conjunctival drug permeability: Systematic comparison and pharmacokinetic impact in the eye. Eur. J. Pharm Sci. 2018, 119, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, Y.H.; Ma, X.W.; Che, J.; Li, C.; Liu, J.; Chen, S.Z.; Wang, Y.Q.; Gan, Y.L.; Chen, H.; Hu, Z.B.; et al. Nanomicelle-assisted targeted ocular delivery with enhanced anti-inflammatory efficacy in vivo. Adv. Sci. 2018, 5, 1700455. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Topical application of polymeric nanomicelles in ophthalmology: A review on research efforts for the non-invasive delivery of ocular therapeutics. Expert Opin. Drug Deliv. 2019, 16, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Surplus micelles for acyclovir ocular delivery: Formulation and cornea and sclera permeability. Int. J. Pharm. 2018, 552, 39–47. [Google Scholar] [CrossRef]
- Agarwal, P.; Rupenthal, I.D. In vitro and ex vivo corneal penetration and absorption models. Drug Deliv. Transl. Res. 2016, 6, 634–647. [Google Scholar] [CrossRef]
- Loch, C.; Zakelj, S.; Kristl, A.; Nagel, S.; Guthoff, R.; Weitschies, W.; Seidlitz, A. Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. Eur. J. Pharm. Sci. 2012, 47, 131–138. [Google Scholar] [CrossRef]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration enhancers in ocular drug delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef] [Green Version]
- OECD Guideline for Testing of Chemicals. Bovine Corneal Opacity and Permeability Test Method for Identifying I) Chemicals Inducing Serious Eye Damage and II) Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage TG 437; OECD: Paris, France, 2015; pp. 1–27. [Google Scholar]
- Ambati, J.; Canakis, C.S.; Miller, J.W.; Gragoudas, E.S.; Edwards, A.; Weissgold DJKim, I.; Delori, F.C.; Adamis, A.P. Diffusion of high molecular weight compounds through sclera. Invest. Ophthalmol. Vis. Sci. 2000, 41, 1181–1185. [Google Scholar]
- Lorenzo-Veiga, B.; Sigurdsson, H.H.; Loftsson, T.; Alvarez-Lorenzo, C. Cyclodextrin–amphiphilic copolymer supramolecular assemblies for the ocular delivery of natamycin. Nanomaterials 2019, 9, 745. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Rivera, F.; Fernández-Villanueva, D.; Concheiro, A.; Alvarez-Lorenzo, C. α-Lipoic acid in soluplus® polymeric nanomicelles for ocular treatment of diabetes-associated corneal diseases. J. Pharm. Sci. 2016, 105, 2855–2863. [Google Scholar] [CrossRef] [Green Version]
- In Vitro Ocular Evaluation Report (ICCVAM). Test Method Evaluation Report: Current Validation Status of In Vitro Test Methods Proposed for Identifying Eye Injury Hazard Potential of Chemicals and Products; National Toxicology Program: Research Triangle Park, NC, USA, 2010; pp. 10–755.
- Sebastián-Morelló, M.; Calatayud-Pascual, M.A.; Rodilla, V.; Balaguer-Fernández, C.; López-Castellano, A. Ex vivo rabbit cornea diffusion studies with a soluble insert of moxifloxacin. Drug Deliv. Transl. Res. 2018, 8, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.C.M.; Nappinnai, M.; Raju, S.; Rao, U.M.V.; Reddy, V.B. Fluconazole ocular inserts: Formulation and in-vitro evaluation. J. Pharm. Sci. Res. 2010, 2, 693–699. [Google Scholar]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Rodrigues, L.B.; Bravo, R.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.; et al. Bimatoprost-loaded ocular inserts as sustained release drug delivery systems for glaucoma treatment: In vitro and in vivo evaluation. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, M.A.; Nicoli, S.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C. Cyclosporine-loaded cross-linked inserts of sodium hyaluronan and hydroxypropyl-β-cyclodextrin for ocular administration. Carbohydr. Polym. 2018, 201, 308–316. [Google Scholar] [CrossRef]
- Dave, V.; Paliwal, S.; Yadav, S.; Sharma, S. Effect of in vitro transcorneal approach of aceclofenac eye drops through excised goat, sheep, and buffalo corneas. Sci. World J. 2015, 2015, 432376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klang, V.; Matsko, N.; Zimmermann, A.M.; Vojnikovic, E.; Valenta, C. Enhancement of stability and skin permeation by sucrose stearate and cyclodextrins in progesterone nanoemulsions. Int. J. Pharm. 2010, 393, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, M.; Dhake, A.S.; Majumdar, D.K. Effect of formulation factors on in-vitro permeation of diclofenac from experimental and marketed aqueous eye drops through excised goat cornea. J. Pharm. Soc. Jpn. 2006, 126, 1369–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BASF. Technical Information Soluplus; BASF: Ludwigshafen am Rhein, Germany, 2010; pp. 1–8. [Google Scholar]
- Daxer, A.; Blumthaler, M.; Schreder, J.; Ettl, A. Effectiveness of eye drops protective against ultraviolet radiation. Ophthalmic Res. 1998, 30, 286–290. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef]
- Nandi, I.; Bateson, M.; Bari, M.; Joshi, H.N. Synergistic effect of PEG-400 and cyclodextrin to enhance solubility of progesterone. AAPS Pharm. Sci. Tech. 2004, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Gidwani, B.; Vyas, A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. Biomed. Res. Int. 2015, 198268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [Green Version]
- Awwad, S.; Mohamed Ahmed, A.H.A.; Sharma, G.; Heng, J.S.; Khaw, P.T.; Brocchini, S.; Lockwood, A. Principles of pharmacology in the eye. Br. J. Pharmacol. 2017, 174, 4205–4223. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Q.; Chuah, K.S.; MacDonald, E.C.A.; Trusler, J.P.M.; Ramaesh, K. The effect of pH, dilution, and temperature on the viscosity of ocular lubricants-shift in rheological parameters and potential clinical significance. Eye 2012, 26, 1579–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Butt, U.; ElShaer, A.; Snyder, L.; Al-Kinani, A.; Le Gresley, A.; Alany, R. Fatty acid based microemulsions to combat ophthalmia neonatorum caused by Neisseria gonorrhoeae and Staphylococcus aureus. Nanomaterials 2018, 8, 51. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, B.; Kay, G.; Matthews, K.H.; Knott, R.M.; Cairns, D. The hen’s egg chorioallantoic membrane (HET-CAM) test to predict the ophthalmic irritation potential of a cysteamine-containing gel: Quantification using Photoshop® and ImageJ. Int. J. Pharm. 2015, 490, 1–8. [Google Scholar] [CrossRef]
- Fathalla, Z.M.A.; Vangala, A.; Longman, M.; Khaled, K.A.; Hussein, A.K.; El-Garhy, O.H.; Alany, R.G. Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: Design, characterisation, toxicity and transcorneal permeation studies. Eur. J. Pharm. Biopharm. 2017, 114, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Nicoli, S.; Ferrari, G.; Quarta, M.; Macaluso, C.; Govoni, P.; Dallatana, D.; Santi, P. Porcine sclera as a model of human sclera for in vitro transport experiments: Histology, SEM, and comparative permeability. Mol. Vis. 2009, 15, 259–266. [Google Scholar]
- Souza, J.G.; Dias, K.; Pereira, T.A.; Bernardi, D.S.; Lopez, R.F.V. Topical delivery of ocular therapeutics: Carrier systems and physical methods. J. Pharm. Pharmacol. 2014, 66, 507–530. [Google Scholar] [CrossRef]
- Savla, R.; Browne, J.; Plassat, V.; Wasan, K.M.; Wasan, E.K. Review and analysis of FDA approved drugs using lipid-based formulations. Drug Dev. Ind. Pharm. 2017, 43, 1743–1758. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Amo, E.M.; Rimpelä, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Bachu, R.D.; Stepanski, M.; Alzhrani, R.M.; Jung, R.; Boddu, S.H.S. Development and evaluation of a novel microemulsion of dexamethasone and tobramycin for topical ocular administration. J. Ocul. Pharmacol. Ther. 2018, 34, 312–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Rivera, F.; Concheiro, A.; Alvarez-Lorenzo, C. Epalrestat-loaded silicone hydrogels as contact lenses to address diabetic-eye complications. Eur. J. Pharm. Biopharm. 2018, 122, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.R.; Maulvi, F.A.; Pandya, M.M.; Ranch, K.M.; Vyas, B.A.; Shah, S.A.; Shah, D.O. Co-delivery of timolol and hyaluronic acid from semi-circular ring-implanted contact lenses for the treatment of glaucoma: In vitro and in vivo evaluation. Biomater. Sci. 2018, 6, 1580–1591. [Google Scholar] [CrossRef]
- Ghosn, M.G.; Tuchin, V.V.; Larin, K.V. Nondestructive quantification of analyte diffusion in cornea and sclera using optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2726–2733. [Google Scholar] [CrossRef] [Green Version]
- Aburahma, M.H.; Mahmoud, A.A. Biodegradable ocular inserts for sustained delivery of brimonidine tartarate: Preparation and in vitro/in vivo evaluation. AAPS Pharm. Sci. Tech. 2011, 12, 1335–1347. [Google Scholar] [CrossRef] [Green Version]






| Formulation | pH | Particle Size (nm) | PDI | Z-Potential (mV) |
|---|---|---|---|---|
| Soluplus 12% | 6.46 | 52.32 ± 10.13 | 0.24 ± 0.01 | −1.17 ± 0.38 |
| Soluplus 12% + PG | 6.38 | 59.19 ± 0.41 | 0.24 ± 0.02 | −1.67 ± 0.62 |
| Pluronic F68 12% | 7.39 | 3.70 ± 1.01 | 0.74 ± 0.08 | −1.76 ± 1.50 |
| Pluronic F68 12% + PG | 7.30 | 3.52 ± 0.15 | 0.73 ± 0.07 | −0.46 ± 0.50 |
| Copolymer (% w/w) | SP (M) | PG (M) | PG (µg/mL) | χ | log P | PM | ∆G for PM (KJ/mol) | mf |
|---|---|---|---|---|---|---|---|---|
| 4 | 3.47 × 10−4 | 2.74 × 10−4 | 86.25 | 7.89 × 10−1 | 4.29 | 56,163,941.1 | −44,211.8 | 0.99995 |
| 8 | 6.95 × 10−4 | 4.74 × 10−4 | 149.12 | 6.82 × 10−1 | 4.53 | 48,546,060.2 | −43,850.6 | 0.99997 |
| 12 | 1.04 × 10−3 | 6.88 × 10−4 | 216.50 | 6.60 × 10−1 | 4.69 | 46,987,265.0 | −43,769.7 | 0.99998 |
| 16 | 1.39 × 10−3 | 8.13 × 10−4 | 255.77 | 5.85 × 10−1 | 4.76 | 41,631,784.1 | −43,469.9 | 0.99998 |
| 20 | 1.73 × 10−3 | 8.21 × 10−4 | 258.18 | 4.72 × 10−1 | 4.77 | 33,619,107.1 | −42,940.3 | 0.99998 |
| Copolymer (% w/w) | PL F68 (M) | PG(M) | PG (µg/mL) | χ | log P | PM | ∆G for PM (KJ/mol) | mf |
|---|---|---|---|---|---|---|---|---|
| 4 | 4.79 × 10−3 | 3.07 × 10−5 | 9.67 | 6.47 × 10−3 | 3.34 | 460,565.1 | −32,309.9 | 0.99954 |
| 8 | 9.58 × 10−3 | 3.52 × 10−5 | 11.08 | 3.69 × 10−3 | 3.40 | 262,942.2 | −30,921.1 | 0.99960 |
| 12 | 1.44 × 10−2 | 5.47 × 10−5 | 17.21 | 3.82 × 10−3 | 3.59 | 271,837.0 | −31,003.5 | 0.99974 |
| 16 | 1.92 × 10−2 | 9.60 × 10−5 | 30.18 | 5.02 × 10−3 | 3.83 | 357,388.1 | −31,681.5 | 0.99985 |
| 20 | 2.39 × 10−2 | 1.49 × 10−5 | 46.81 | 6.23 × 10−3 | 4.02 | 443,288.4 | −32,215.2 | 0.99991 |
| Micelles | Accumulated Amounts of PG in Receptor Compartments (μg/cm2) | |||||
|---|---|---|---|---|---|---|
| Cornea | Sclera | |||||
| Rabbit | Porcine | Bovine | Rabbit | Porcine | Bovine | |
| Soluplus | 3.65 ± 0.50 | 2.44 ± 0.17 | 1.13 ± 0.09 | 2.30 ± 0.64 | 1.12 ± 0.36 | 0.83 ± 0.02 |
| Pluronic | 1.75 ± 0.48 | 0.77 ± 0.13 | 0.92 ± 0.06 | 0.71 ± 0.13 | 0.36 ± 0.23 | 0.82 ± 0.03 |
| Animal | Soluplus/Cornea | Soluplus/Sclera | Pluronic/Cornea | Pluronic/Sclera | ||||
|---|---|---|---|---|---|---|---|---|
| J | Papp (×107) | J | Papp (×107) | J | Papp (×107) | J | Papp (×107) | |
| Rabbit | 1.38 ± 0.15 | 16.5 ± 1.8 | 0.77 ± 0.17 | 9.2 ± 2.0 | 0.66 ± 0.19 | 37.3 ± 10.5 | 0.26 ± 0.03 | 14.5 ± 1.5 |
| Pig | 0.91 ± 0.04 | 9.8 ± 0.5 | 0.37 ± 0.08 | 4.0 ± 0.9 | 0.26 ± 0.04 | 15.2 ± 2.3 | 0.09 ± 0.07 | 5.6 ± 3.9 |
| Cow | 0.31 ± 0.07 | 3.4 ± 0.8 | 0.11 ± 0.01 | 1.2 ± 0.1 | 0.10 ± 0.03 | 6.1 ± 1.5 | 0.11 ± 0.01 | 6.8 ± 0.8 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alambiaga-Caravaca, A.M.; Calatayud-Pascual, M.A.; Rodilla, V.; Concheiro, A.; López-Castellano, A.; Alvarez-Lorenzo, C. Micelles of Progesterone for Topical Eye Administration: Interspecies and Intertissues Differences in Ex Vivo Ocular Permeability. Pharmaceutics 2020, 12, 702. https://doi.org/10.3390/pharmaceutics12080702
Alambiaga-Caravaca AM, Calatayud-Pascual MA, Rodilla V, Concheiro A, López-Castellano A, Alvarez-Lorenzo C. Micelles of Progesterone for Topical Eye Administration: Interspecies and Intertissues Differences in Ex Vivo Ocular Permeability. Pharmaceutics. 2020; 12(8):702. https://doi.org/10.3390/pharmaceutics12080702
Chicago/Turabian StyleAlambiaga-Caravaca, Adrián M., María Aracely Calatayud-Pascual, Vicent Rodilla, Angel Concheiro, Alicia López-Castellano, and Carmen Alvarez-Lorenzo. 2020. "Micelles of Progesterone for Topical Eye Administration: Interspecies and Intertissues Differences in Ex Vivo Ocular Permeability" Pharmaceutics 12, no. 8: 702. https://doi.org/10.3390/pharmaceutics12080702
APA StyleAlambiaga-Caravaca, A. M., Calatayud-Pascual, M. A., Rodilla, V., Concheiro, A., López-Castellano, A., & Alvarez-Lorenzo, C. (2020). Micelles of Progesterone for Topical Eye Administration: Interspecies and Intertissues Differences in Ex Vivo Ocular Permeability. Pharmaceutics, 12(8), 702. https://doi.org/10.3390/pharmaceutics12080702