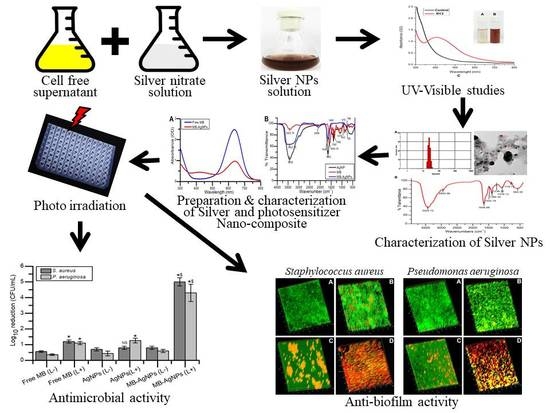
Biogenic Silver Nanoparticles Decorated with Methylene Blue Potentiated the Photodynamic Inactivation of Pseudomonas aeruginosa and Staphylococcus aureus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Test Bacteria and Growth Conditions
2.3. Isolation and Identification of Bacterial Isolates
2.4. Extracellular Synthesis of AgNPs
2.5. Characterization of AgNPs
2.5.1. UV-Visible (UV-Vis) Spectroscopy
2.5.2. Particle Size and Transmission Electron Microscopic (TEM) Analysis
2.5.3. Fourier Transform Infrared Spectroscopic Analysis (FTIR)
2.5.4. X-ray Powder Diffraction (XRD) and SEM-EDX Analysis
2.6. Synthesis of MB-AgNPs
2.7. Characterization of MB-AgNPs
2.8. In Vitro Release of MB
2.9. Methylene Blue Uptake
2.10. Light Source
2.11. Minimum Inhibitory Concentration (MIC)
2.12. Antimicrobial Photodynamic Inactivation of Bacterial Cells
2.13. Detection of ROS
2.14. Protein Leakage
2.15. Biofilm Inhibition Assay
2.16. Confocal Laser Scanning Microscopy (CLSM)
2.17. Statistical Analysis
3. Results
3.1. Isolation and Identification of Bacteria
3.2. Synthesis and Characterization of AgNPs
3.3. Preparation and Characterization of MB-AgNPs
3.4. In Vitro Release and Bacterial Uptake Study
3.5. Minimum Inhibitory Concentration (MIC)
3.6. aPDT on Planktonic Cells
3.7. Detection of ROS
3.8. Protein Leakage
3.9. Biofilm Formation
3.10. Microscopic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mendes, F.; Paulo, A.; Santos, I. Metalloprobes for functional monitoring of tumour multidrug resistance by nuclear imaging. Dalton Trans. 2011, 40, 5377–5393. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, B.; Pei, X.; Shangguan, F. Multiplex analysis of tumor multidrug-resistance genes expression with photonic suspension array. Analyst 2012, 137, 3343–3348. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Lyutakov, O.; Hejna, O.; Solovyev, A.; Kalachyova, Y.; Svorcik, V. Polymethylmethacrylate doped with porphyrin and silver nanoparticles as light-activated antimicrobial material. RSC Adv. 2014, 4, 50624–50630. [Google Scholar] [CrossRef]
- Maiti, S.; Krishnan, D.; Barman, G.; Ghosh, S.K.; Laha, J.K. Antimicrobial activities of silver nanoparticles synthesized from Lycopersicon esculentum extract. J. Anal. Sci. Technol. 2014, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Predoi, D.; Popa, C.; Chapon, P.; Groza, A.; Iconaru, S. Evaluation of the antimicrobial activity of different antibiotics enhanced with silver-doped hydroxyapatite thin films. Materials 2016, 9, 778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Naik, A.J.T.; Ismail, S.; Kay, C.; Wilson, M.; Parkin, I.P. Antimicrobial activity of polyurethane embedded with methylene blue, toluidene blue and gold nanoparticles against Staphylococcus aureus; illuminated with white light. Mater. Chem. Phys. 2011, 129, 446–450. [Google Scholar] [CrossRef]
- Hanakova, A.; Bogdanova, K.; Tomankova, K.; Pizova, K.; Malohlava, J.; Binder, S.; Bajgar, R.; Langova, K.; Kolar, M.; Mosinger, J.; et al. The application of antimicrobial photodynamic therapy on S. aureus and E. coli using porphyrin photosensitizers bound to cyclodextrin. Microbiol. Res. 2014, 169, 163–170. [Google Scholar] [CrossRef]
- Wu, J.; Xu, H.; Tang, W.; Kopelman, R.; Philbert, M.A.; Xi, C. Eradication of bacteria in suspension and biofilms using methylene blue-loaded dynamic nanoplatforms. Antimicrob. Agents Chemother. 2009, 53, 3042–3048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Khan, S.N.; Meena, R.; Dar, A.M.; Pal, R.; Khan, A.U. Photoinactivation of multidrug resistant bacteria by monomeric methylene blue conjugated gold nanoparticles. J. Photochem. Photobiol. B Biol. 2017, 174, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, M.V.; Borghi-Pangoni, F.B.; Ferreira, S.B.S.; Rabello, B.R.; Hioka, N.; Bruschi, M.L. Functional polymeric systems as delivery vehicles for methylene blue in photodynamic therapy. Langmuir 2016, 32, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Misba, L.; Kulshrestha, S.; Khan, A.U. Antibiofilm action of a toluidine blue O-silver nanoparticle conjugate on Streptococcus mutans: A mechanism of type I photodynamic therapy. Biofouling 2016, 32, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, L.; Chen, J.; Liu, W.; Zhang, D.; Xu, P.; Dai, T.; Shang, L.; Yang, Y.; Tang, S.; et al. Composite of silver nanoparticles and photosensitizer leads to mutual enhancement of antimicrobial efficacy and promotes wound healing. Chem. Eng. J. 2019, 374, 1373–1381. [Google Scholar] [CrossRef]
- Tawfik, A.A.; Noaman, I.; El-Elsayyad, H.; El-Mashad, N.; Soliman, M. A study of the treatment of cutaneous fungal infection in animal model using photoactivated composite of methylene blue and gold nanoparticle. Photodiagnosis Photodyn. Ther. 2016, 15, 59–69. [Google Scholar] [CrossRef]
- Tunçel, A.; Öztürk, İ.; Ince, M.; Ocakoglu, K.; Hoşgör-Limoncu, M.; Yurt, F. Antimicrobial photodynamic therapy against Staphylococcus aureus using zinc phthalocyanine and zinc phthalocyanine-integrated TiO2 nanoparticles. J. Porphyr. Phthalocyanines 2019, 23, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Sherwani, M.A.; Tufail, S.; Khan, A.A.; Owais, M. Gold nanoparticle-photosensitizer conjugate based photodynamic inactivation of biofilm producing cells: Potential for treatment of C. albicans infection in BALB/c mice. PLoS ONE 2015, 10, e0131684. [Google Scholar] [CrossRef]
- Khan, S.; Alam, F.; Azam, A.; Khan, A.U. Gold nanoparticles enhance methylene blue-induced photodynamic therapy: A novel therapeutic approach to inhibit Candida albicans biofilm. Int. J. Nanomedicine 2012, 7, 3245–3257. [Google Scholar] [CrossRef] [Green Version]
- Das, V.L.; Thomas, R.; Varghese, R.T.; Soniya, E.V.; Mathew, J.; Radhakrishnan, E.K. Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech 2014, 4, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.-Y.; Narsing Rao, M.P.; Xiao, M.; Wang, H.-F.; Hozzein, W.N.; Chen, W.; Li, W.-J. Antibacterial Activity of Silver nanoparticles against Staphylococcus warneri synthesized using endophytic bacteria by photo-irradiation. Front. Microbiol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Shivaji, S.; Madhu, S.; Singh, S. Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem. 2011, 46, 1800–1807. [Google Scholar] [CrossRef]
- Jain, N.; Bhargava, A.; Majumdar, S.; Tarafdar, J.C.; Panwar, J. Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: A mechanism perspective. Nanoscale 2011, 3, 635–641. [Google Scholar] [CrossRef]
- Raju, D.; Paneliya, N.; Mehta, U.J. Extracellular synthesis of silver nanoparticles using living peanut seedling. Appl. Nanosci. 2014, 4, 875–879. [Google Scholar] [CrossRef] [Green Version]
- AbdelRahim, K.; Mahmoud, S.Y.; Ali, A.M.; Almaary, K.S.; Mustafa, A.E.-Z.M.A.; Husseiny, S.M. Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi J. Biol. Sci. 2017, 24, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Deobagkar, D.; Kulkarni, R.; Shaiwale, N.; Deobagkar, D. Synthesis and extracellular accumulation of silver nanoparticles by employing radiation-resistant Deinococcus radiodurans, their characterization, and determination of bioactivity. Int. J. Nanomed. 2015, 10, 963–974. [Google Scholar] [CrossRef] [Green Version]
- Usacheva, M.; Layek, B.; Rahman Nirzhor, S.S.; Prabha, S. Nanoparticle-mediated photodynamic therapy for mixed biofilms. J. Nanomater. 2016. [Google Scholar] [CrossRef] [Green Version]
- Rolim, J.P.M.L.; De-Melo, M.A.S.; Guedes, S.F.; Albuquerque-Filho, F.B.; de Souza, J.R.; Nogueira, N.A.P.; Zanin, I.C.J.; Rodrigues, L.K.A. The antimicrobial activity of photodynamic therapy against Streptococcus mutans using different photosensitizers. J. Photochem. Photobiol. B Biol. 2012, 106, 40–46. [Google Scholar] [CrossRef]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2015, 10, 862–876. [Google Scholar] [CrossRef] [Green Version]
- Ichinose-Tsuno, A.; Aoki, A.; Takeuchi, Y.; Kirikae, T.; Shimbo, T.; Lee, M.-C.; Yoshino, F.; Maruoka, Y.; Itoh, T.; Ishikawa, I.; et al. Antimicrobial photodynamic therapy suppresses dental plaque formation in healthy adults: A randomized controlled clinical trial. BMC Oral Health 2014, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Erbas, S.; Gorgulu, A.; Kocakusakogullari, M.; Akkaya, E.U. Non-covalent functionalized SWNTs as delivery agents for novel Bodipy-based potential PDT sensitizers. Chem. Commun. 2009, 4956–4958. [Google Scholar] [CrossRef] [PubMed]
- Thombre, R.S.; Shinde, V.; Thaiparambil, E.; Zende, S.; Mehta, S. Antimicrobial activity and mechanism of inhibition of silver nanoparticles against extreme halophilic archaea. Front. Microbiol. 2016, 7, 1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- de Melo, W.C.; Avci, P.; de Oliveira, M.N.; Gupta, A.; Vecchio, D.; Sadasivam, M.; Chandran, R.; Huang, Y.-Y.; Yin, R.; Perussi, L.R.; et al. Photodynamic inactivation of biofilm: Taking a lightly colored approach to stubborn infection. Expert Rev. Anti. Infect. Ther. 2013, 11, 669–693. [Google Scholar] [CrossRef] [Green Version]
- Cieplik, F.; Tabenski, L.; Buchalla, W.; Maisch, T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front. Microbiol. 2014, 5, 405. [Google Scholar] [CrossRef] [Green Version]
- Predoi, D.; Iconaru, S.; Predoi, M. Bioceramic layers with antifungal properties. Coatings 2018, 8, 276. [Google Scholar] [CrossRef] [Green Version]
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef]
- Anju, V.T.; Paramanantham, P.; Siddhardha, B.; Lal, S.S.; Sharan, A.; Alyousef, A.A.; Arshad, M.; Syed, A. Malachite green-conjugated multi-walled carbon nanotubes potentiate antimicrobial photodynamic inactivation of planktonic cells and biofilms of Pseudomonas aeruginosa and Staphylococcus aureus. Int. J. Nanomed. 2019, 14, 3861–3874. [Google Scholar] [CrossRef] [Green Version]
- Tawfik, A.A.; Alsharnoubi, J.; Morsy, M. Photodynamic antibacterial enhanced effect of methylene blue-gold nanoparticles conjugate on Staphylococcal aureus isolated from impetigo lesions in vitro study. Photodiagnosis Photodyn. Ther. 2015, 12, 215–220. [Google Scholar] [CrossRef]
- Płaza, G.A.; Chojniak, J.; Mendrek, B.; Trzebicka, B.; Kvitek, L.; Panacek, A.; Prucek, R.; Zboril, R.; Paraszkiewicz, K.; Bernat, P. Synthesis of silver nanoparticles by Bacillus subtilis T-1 growing on agro-industrial wastes and producing biosurfactant. IET Nanobiotechnol. 2016, 10, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Jasuja, N.D.; Kumar, M.; Ali, M.I. Biological synthesis of silver nanoparticles by cell-free extract of Spirulina platensis. J. Nanotechnol. 2015. [Google Scholar] [CrossRef] [Green Version]
- Fouad, H.; Li, H.J.; Ding, Y.M.; Yu, B.T.; El-Shakh, A.; Ghulam, A.; Mo, J.C. Synthesis and characterization of silver nanoparticles using Bacillus amyloliquefaciens and Bacillus subtilis to control filarial vector Culex pipiens pallens and its antimicrobial activity. Artif. Cells Nanomedici. Biotechnol. 2017, 45, 1369–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunkar, S.; Nachiyar, C.V. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac. J. Trop. Biomed. 2012, 2, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Rudakiya, D.M.; Pawar, K. Bactericidal potential of silver nanoparticles synthesized using cell-free extract of Comamonas acidovorans: In vitro and in silico approaches. 3 Biotech 2017, 7, 92. [Google Scholar] [CrossRef] [Green Version]
- Iconaru, S.L.; Chapon, P.; Le Coustumer, P.; Predoi, D. Antimicrobial activity of thin solid films of silver doped hydroxyapatite prepared by sol-gel method. Sci. World J. 2014. [Google Scholar] [CrossRef] [Green Version]
- Planas, O.; Bresolí-Obach, R.; Nos, J.; Gallavardin, T.; Ruiz-González, R.; Agut, M.; Nonell, S. Synthesis, photophysical characterization, and photoinduced antibacterial activity of methylene blue-loaded amino- and mannose-targeted mesoporous silica nanoparticles. Molecules 2015, 20, 6284–6298. [Google Scholar] [CrossRef] [Green Version]
- Amin, A.; Kaduskar, D.V. Comparative study on photodynamic activation of ortho-toluidine blue and methylene blue loaded mesoporous silica nanoparticles against resistant microorganisms. Recent Pat. Drug Deliv. Formul. 2019, 12, 154–161. [Google Scholar] [CrossRef]
- Darabpour, E.; Kashef, N.; Mashayekhan, S. Chitosan nanoparticles enhance the efficiency of methylene blue-mediated antimicrobial photodynamic inactivation of bacterial biofilms: An in vitro study. Photodiagnosis Photodyn. Ther. 2016, 14, 211–217. [Google Scholar] [CrossRef]
- Grinholc, M.; Nakonieczna, J.; Fila, G.; Taraszkiewicz, A.; Kawiak, A.; Szewczyk, G.; Sarna, T.; Lilge, L.; Bielawski, K.P. Antimicrobial photodynamic therapy with fulleropyrrolidine: Photoinactivation mechanism of Staphylococcus aureus, in vitro and in vivo studies. Appl. Microbiol. Biotechnol. 2015, 99, 4031–4043. [Google Scholar] [CrossRef] [Green Version]
- George, S.; Kishen, A. Influence of photosensitizer solvent on the mechanisms of photoactivated killing of Enterococcus faecalis. Photochem. Photobiol. 2008, 84, 734–740. [Google Scholar] [CrossRef]
- Misba, L.; Zaidi, S.; Khan, A.U. A comparison of antibacterial and antibiofilm efficacy of phenothiazinium dyes between Gram positive and Gram negative bacterial biofilm. Photodiagnosis Photodyn. Ther. 2017, 18, 24–33. [Google Scholar] [CrossRef]
- Paramanantham, P.; Siddhardha, B.; Sruthil Lal, S.B.; Sharan, A.; Alyousef, A.A.; Al Dosary, M.S.; Arshad, M.; Syed, A. Antimicrobial photodynamic therapy on Staphylococcus aureus and Escherichia coli using malachite green encapsulated mesoporous silica nanoparticles: An in vitro study. PeerJ 2019, 7, e7454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mang, T.; Rogers, S.; Keinan, D.; Honma, K.; Baier, R. Antimicrobial photodynamic therapy (aPDT) induction of biofilm matrix architectural and bioadhesive modifications. Photodiagnosis Photodyn. Ther. 2016, 13, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, P.; Antony, A.P.; Sruthil Lal, S.B.; Sharan, A.; Siddhardha, B.; Kasinathan, K.; Bahkali, N.A.; Dawoud, T.M.S.; Syed, A. Antimicrobial photodynamic activity of toluidine blue encapsulated in mesoporous silica nanoparticles against Pseudomonas aeruginosa and Staphylococcus aureus. Biofouling 2019, 35, 89–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klepac-Ceraj, V.; Patel, N.; Song, X.; Holewa, C.; Patel, C.; Kent, R.; Amiji, M.M.; Soukos, N.S. Photodynamic effects of methylene blue-loaded polymeric nanoparticles on dental plaque bacteria. Lasers Surg. Med. 2011, 43, 600–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parasuraman, P.; R. Y, T.; Shaji, C.; Sharan, A.; Bahkali, A.H.; Al-Harthi, H.F.; Syed, A.; Anju, V.T.; Dyavaiah, M.; Siddhardha, B. Biogenic Silver Nanoparticles Decorated with Methylene Blue Potentiated the Photodynamic Inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Pharmaceutics 2020, 12, 709. https://doi.org/10.3390/pharmaceutics12080709
Parasuraman P, R. Y T, Shaji C, Sharan A, Bahkali AH, Al-Harthi HF, Syed A, Anju VT, Dyavaiah M, Siddhardha B. Biogenic Silver Nanoparticles Decorated with Methylene Blue Potentiated the Photodynamic Inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Pharmaceutics. 2020; 12(8):709. https://doi.org/10.3390/pharmaceutics12080709
Chicago/Turabian StyleParasuraman, Paramanantham, Thamanna R. Y, Chitra Shaji, Alok Sharan, Ali H. Bahkali, Helal F. Al-Harthi, Asad Syed, V.T. Anju, Madhu Dyavaiah, and Busi Siddhardha. 2020. "Biogenic Silver Nanoparticles Decorated with Methylene Blue Potentiated the Photodynamic Inactivation of Pseudomonas aeruginosa and Staphylococcus aureus" Pharmaceutics 12, no. 8: 709. https://doi.org/10.3390/pharmaceutics12080709
APA StyleParasuraman, P., R. Y, T., Shaji, C., Sharan, A., Bahkali, A. H., Al-Harthi, H. F., Syed, A., Anju, V. T., Dyavaiah, M., & Siddhardha, B. (2020). Biogenic Silver Nanoparticles Decorated with Methylene Blue Potentiated the Photodynamic Inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Pharmaceutics, 12(8), 709. https://doi.org/10.3390/pharmaceutics12080709