Chitosan/Polycyclodextrin (CHT/PCD)-Based Sponges Delivering VEGF to Enhance Angiogenesis for Bone Regeneration
Abstract
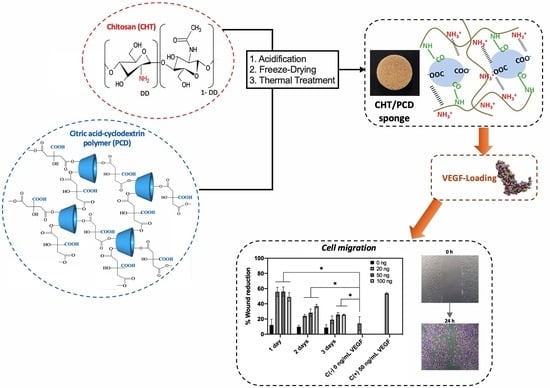
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of CHT/PCD Sponges
2.2.2. Characterization of Macroporous Hydrogels
Microstructure
- Scanning Electron Microscopy (SEM)
- X-Ray Microtomography (μCT) Imaging and Quantitative Analysis
- Water Uptake
- In Vitro Biodegradation
Mechanical Property
2.2.3. Cytocompatibility
Cell Culture
Cell Viability
LIVE/DEAD® Assay
2.2.4. VEGF-Loading on CHT/PCD Sponges
2.2.5. In Vitro VEGF Release
2.2.6. Bioactivity of Released VEGF from Sponges
HUVECs Proliferation Assay
HUVECs Migration Assay
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Macroporous Hydrogels
3.1.1. Microstructure
3.1.2. Water Uptake
3.1.3. Biodegradation
3.1.4. Mechanical Property
3.2. Cytocompatibility
3.3. In Vitro VEGF Release
3.4. Bioactivity of Released VEGF
3.4.1. HUVECs Proliferation Assay
3.4.2. HUVECs Migration Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lindahl, A.; Brittberg, M.; Gibbs, D.; Dawson, J.I.; Kanczler, J.; Black, C.; Tare, R.; Oreffo, R.O.C. Cartilage and Bone Regeneration. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 529–582. ISBN 978-0-12-420145-3. [Google Scholar]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, M.; Schmidt-Bleek, K.; Duda, G.N.; Mooney, D.J. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv. Drug Deliv. Rev. 2012, 64, 1257–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K.; Kandalam, U. Three-Dimensional macroporous materials for tissue engineering of craniofacial bone. Br. J. Oral Maxillofac. Surg. 2017, 55, 875–891. [Google Scholar] [CrossRef] [PubMed]
- Tollemar, V.; Collier, Z.J.; Mohammed, M.K.; Lee, M.J.; Ameer, G.A.; Reid, R.R. Stem cells, growth factors and scaffolds in craniofacial regenerative medicine. Genes Dis. 2016, 3, 56–71. [Google Scholar] [CrossRef] [Green Version]
- Akter, F.; Ibanez, J. Bone and Cartilage Tissue Engineering. In Tissue Engineering Made Easy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 77–97. ISBN 978-0-12-805361-4. [Google Scholar]
- Lin, Y.; Huang, S.; Zou, R.; Gao, X.; Ruan, J.; Weir, M.D.; Reynolds, M.A.; Qin, W.; Chang, X.; Fu, H.; et al. Calcium phosphate cement scaffold with stem cell co-culture and prevascularization for dental and craniofacial bone tissue engineering. Dent. Mater. 2019, 35, 1031–1041. [Google Scholar] [CrossRef]
- Boontheekul, T.; Mooney, D.J. Protein-based signaling systems in tissue engineering. Curr. Opin. Biotechnol. 2003, 14, 559–565. [Google Scholar] [CrossRef]
- Fu, J.; Wang, D.-A. In Situ Organ-Specific Vascularization in Tissue Engineering. Trends Biotechnol. 2018, 36, 834–849. [Google Scholar] [CrossRef]
- De Rosa, L.; Di Stasi, R.; D’Andrea, L.D. Pro-angiogenic peptides in biomedicine. Arch. Biochem. Biophys. 2018, 660, 72–86. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Beamer, B.; Hettrich, C.; Lane, J. Vascular Endothelial Growth Factor: An Essential Component of Angiogenesis and Fracture Healing. HSS J. 2009, 6, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carulli, C.; Innocenti, M.; Brandi, M.L. Bone Vascularization in Normal and Disease Conditions. Front. Endocrinol. 2013, 4, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Riva, B.; Nowak, C.; Sánchez, E.; Hernández, A.; Schulz-Siegmund, M.; Pec, M.K.; Delgado, A.; Évora, C. VEGF-Controlled release within a bone defect from alginate/chitosan/PLA-H scaffolds. Eur. J. Pharm. Biopharm. 2009, 73, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Ai, J.; Hadjati, J.; Azami, M. Bio-Hybrid silk fibroin/calcium phosphate/PLGA nanocomposite scaffold to control the delivery of vascular endothelial growth factor. Mater. Sci. Eng. C 2014, 35, 401–410. [Google Scholar] [CrossRef]
- Echave, M.C.; Pimenta-Lopes, C.; Pedraz, J.L.; Mehrali, M.; Dolatshahi-Pirouz, A.; Ventura, F.; Orive, G. Enzymatic crosslinked gelatin 3D scaffolds for bone tissue engineering. Int. J. Pharm. 2019, 562, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Dou, D.D.; Zhou, G.; Liu, H.W.; Zhang, J.; Liu, M.L.; Xiao, X.F.; Fei, J.J.; Guan, X.L.; Fan, Y.B. Sequential releasing of VEGF and BMP-2 in hydroxyapatite collagen scaffolds for bone tissue engineering: Design and characterization. Int. J. Boil. Macromol. 2019, 123, 622–628. [Google Scholar] [CrossRef]
- Almubarak, S.; Nethercott, H.; Freeberg, M.; Beaudon, C.; Jha, A.; Jackson, W.; Marcucio, R.; Miclau, T.; Healy, K.; Bahney, C. Tissue engineering strategies for promoting vascularized bone regeneration. Bone 2016, 83, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhang, Z.; Ma, X.; Duan, Z.; Hui, J.; Zhu, C.; Zhang, D.; Fan, D.; Shang, L.; Chen, F. Newly Designed Human-Like Collagen to Maximize Sensitive Release of BMP-2 for Remarkable Repairing of Bone Defects. Biomolecules 2019, 9, 450. [Google Scholar] [CrossRef] [Green Version]
- Claaßen, C.; Sewald, L.; Tovar, G.E.M.; Borchers, K. Controlled Release of Vascular Endothelial Growth Factor from Heparin-Functionalized Gelatin Type A and Albumin Hydrogels. Gels 2017, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Suliman, S.; Xing, Z.; Wu, X.; Xue, Y.; Pedersen, T.O.; Sun, Y.; Døskeland, A.P.; Nickel, J.; Waag, T.; Lygre, H.; et al. Release and bioactivity of bone morphogenetic protein-2 are affected by scaffold binding techniques in vitro and in vivo. J. Control. Release 2015, 197, 148–157. [Google Scholar] [CrossRef]
- Chiu, L.L.Y.; Radisic, M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials 2010, 31, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Alarçin, E.; Lee, T.Y.; Karuthedom, S.; Mohammadi, M.; Brennan, M.A.; Lee, D.H.; Marrella, A.; Zhang, J.; Syla, D.; Zhang, Y.S.; et al. Injectable shear-Thinning hydrogels for delivering osteogenic and angiogenic cells and growth factors. Biomater. Sci. 2018, 6, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Behr, B.; Sorkin, M.; Lehnhardt, M.; Renda, A.; Longaker, M.T.; Quarto, N. A Comparative Analysis of the Osteogenic Effects of BMP-2, FGF-2, and VEGFA in a Calvarial Defect Model. Tissue Eng. Part A 2012, 18, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Amirian, J.; Linh, N.T.B.; Min, Y.K.; Lee, B.-T. The effect of BMP-2 and VEGF loading of gelatin-pectin-BCP scaffolds to enhance osteoblast proliferation. J. Appl. Polym. Sci. 2014, 132. [Google Scholar] [CrossRef]
- Dinu, M.V.; Dragan, E.S. Macroporous Hydrogels: Preparation, Properties, and Applications. In Hydrogels; Thakur, V.K., Thakur, M.K., Eds.; Springer: Singapore, 2018; pp. 51–85. ISBN 978-981-10-6076-2. [Google Scholar]
- Flégeau, K.; Pace, R.; Gautier, H.; Rethore, G.; Guicheux, J.; Le Visage, C.; Weiss, P. Toward the development of biomimetic injectable and macroporous biohydrogels for regenerative medicine. Adv. Colloid Interface Sci. 2017, 247, 589–609. [Google Scholar] [CrossRef]
- Berretta, J.; Bumgardner, J.D.; Jennings, J.A. Lyophilized chitosan sponges. In Chitosan Based Biomaterials, Volume 1; Elsevier: Amsterdam, The Netherlands, 2017; pp. 239–253. [Google Scholar]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-Based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan; Jana, S., Ed.; Springer: Singapore, 2019; pp. 457–489. ISBN 9789811502620. [Google Scholar]
- Flores, C.; Lopez, M.; Tabary, N.; Neut, C.; Chai, F.; Betbeder, D.; Herkt, C.; Cazaux, F.; Gaucher, V.; Martel, B.; et al. Preparation and characterization of novel chitosan and β-Cyclodextrin polymer sponges for wound dressing applications. Carbohydr. Polym. 2017, 173, 535–546. [Google Scholar] [CrossRef]
- Palomino-Durand, C.; Lopez, M.; Cazaux, F.; Martel, B.; Blanchemain, N.; Chai, F. Influence of the Soluble⁻Insoluble Ratios of Cyclodextrins Polymers on the Viscoelastic Properties of Injectable Chitosan⁻Based Hydrogels for Biomedical Application. Polymers 2019, 11, 214. [Google Scholar] [CrossRef] [Green Version]
- Martel, B.; Ruffin, D.; Weltrowski, M.; Lekchiri, Y.; Morcellet, M. Water-Soluble polymers and gels from the polycondensation between cyclodextrins and poly(carboxylic acid)s: A study of the preparation parameters. J. Appl. Polym. Sci. 2005, 97, 433–442. [Google Scholar] [CrossRef]
- Garcia-Fernandez, M.J.; Tabary, N.; Chai, F.; Cazaux, F.; Blanchemain, N.; Flament, M.P.; Martel, B. New multifunctional pharmaceutical excipient in tablet formulation based on citric acid-cyclodextrin polymer. Int. J. Pharm. 2016, 511, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Mogrovejo-Valdivia, A.; Rahmouni, O.; Tabary, N.; Maton, M.; Neut, C.; Martel, B.; Blanchemain, N. In vitro evaluation of drug release and antibacterial activity of a silver-Loaded wound dressing coated with a multilayer system. Int. J. Pharm. 2018, 556, 301–310. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Sariibrahimoglu, K.; Yang, W.; Bohner, M.; Yamashita, D.; Kunstar, A.; Van Apeldoorn, A.A.; Bronkhorst, E.M.; Félix Lanao, R.P.; Leeuwenburgh, S.C.G.; et al. Influence of the pore generator on the evolution of the mechanical properties and the porosity and interconnectivity of a calcium phosphate cement. Acta Biomater. 2012, 8, 404–414. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernabe, P.; Peniche, C.; Argüelles-Monal, W. Swelling behavior of chitosan/pectin polyelectrolyte complex membranes. Effect of thermal cross-linking. Polym. Bull. 2005, 55, 367–375. [Google Scholar] [CrossRef]
- Ji, C.; Shi, J. Thermal-Crosslinked porous chitosan scaffolds for soft tissue engineering applications. Mater. Sci. Eng. C 2013, 33, 3780–3785. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Preethi Soundarya, S.; Haritha Menon, A.; Viji Chandran, S.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Boil. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef]
- Felfel, R.; Gideon-Adeniyi, M.J.; Zakir Hossain, K.M.; Roberts, G.A.F.; Grant, D.M. Structural, mechanical and swelling characteristics of 3D scaffolds from chitosan-agarose blends. Carbohydr. Polym. 2019, 204, 59–67. [Google Scholar] [CrossRef]
- Niranjan, R.M.; Koushik, C.; Saravanan, S.; Moorthi, A.; Vairamani, M.; Selvamurugan, N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int. J. Boil. Macromol. 2013, 54, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Jain, Y.; Sinha, S.; Rastogi, A.; Srivastava, P. Design and evaluation of chitosan/chondroitin sulfate/nano-bioglass based composite scaffold for bone tissue engineering. Int. J. Boil. Macromol. 2019, 133, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.A. Controlling chitosan degradation properties in vitro and in vivo. In Chitosan Based Biomaterials, Volume 1; Elsevier: Amsterdam, The Netherlands, 2017; pp. 159–182. [Google Scholar]
- Lončarević, A.; Ivanković, M.; Rogina, A. Lysozyme-Induced Degradation of Chitosan: The Characterisation of Degraded Chitosan Scaffolds. J. Tissue Repair Regen. 2017, 1, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Shamekhi, M.A.; Rabiee, A.; Mirzadeh, H.; Mahdavi, H.; Mohebbi-Kalhori, D.; Baghaban Eslaminejad, M. Fabrication and characterization of hydrothermal cross-linked chitosan porous scaffolds for cartilage tissue engineering applications. Mater. Sci. Eng. C 2017, 80, 532–542. [Google Scholar] [CrossRef]
- Gil, E.S.; Kluge, J.A.; Rockwood, D.N.; Rajkhowa, R.; Wang, L.; Wang, X.; Kaplan, D.L. Mechanical improvements to reinforced porous silk scaffolds. J. Biomed. Mater. Res. Part A 2011, 99A, 16–28. [Google Scholar] [CrossRef] [Green Version]
- Seda Tığlı, R.; Karakeçili, A.; Gümüşderelioğlu, M. In vitro characterization of chitosan scaffolds: Influence of composition and deacetylation degree. J. Mater. Sci. Mater. Electron. 2007, 18, 1665–1674. [Google Scholar] [CrossRef]
- Amiel, A.G.; Palomino-Durand, C.; Maton, M.; Lopez, M.; Cazaux, F.; Chai, F.; Neut, C.; Foligné, B.; Martel, B.; Blanchemain, N. Designed sponges based on chitosan and cyclodextrin polymer for a local release of ciprofloxacin in diabetic foot infections. Int. J. Pharm. 2020, 587, 119677. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, S.; Shao, X.; Zhang, Q.; Xue, C.; Zhang, S.; Lin, Y.; Zhu, B.; Cai, X. Effect of matrix stiffness on osteoblast functionalization. Cell Prolif. 2017, 50, e12338. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Z.-K.; Lai, P.-L.; Toh, E.K.-W.; Weng, C.-H.; Tseng, H.-W.; Chang, P.-Z.; Chen, C.-C.; Cheng, C.-M. Osteogenic differentiation of preosteoblasts on a hemostatic gelatin sponge. Sci. Rep. 2016, 6, 32884. [Google Scholar] [CrossRef] [Green Version]
- Amaral, I.F.; Sampaio, P.; Barbosa, M.A. Three-dimensional culture of human osteoblastic cells in chitosan sponges: The effect of the degree of acetylation. J. Biomed. Mater. Res. Part A 2005, 76, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, A.; Anisha, B.S.; Chennazhi, K.P.; Jayakumar, R. Chitosan–hyaluronic acid/VEGF loaded fibrin nanoparticles composite sponges for enhancing angiogenesis in wounds. Colloids Surf. B: Biointerfaces 2015, 127, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Zhang, M.; Wu, Z.-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010, 31, 6279–6308. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-Based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Blanchemain, N.; Karrout, Y.; Tabary, N.; Bria, M.; Neut, C.; Hildebrand, H.F.; Siepmann, J.; Martel, B. Comparative study of vascular prostheses coated with polycyclodextrins for controlled ciprofloxacin release. Carbohydr. Polym. 2012, 90, 1695–1703. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Zhou, G.; Zhang, J.; Su, X.; Huang, Z.; Li, Q.; Wu, Z.; Qiu, G. VEGF-Loaded biomimetic scaffolds: A promising approach to improve angiogenesis and osteogenesis in an ischemic environment. RSC Adv. 2017, 7, 4253–4259. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, C.R.; Banfi, A.; Glazer, N.L.; Thurston, G.; Springer, M.L.; Kraft, P.E.; McDonald, D.M.; Blau, H.M. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J. Clin. Investig. 2004, 113, 516–527. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.C.; Chen, S.; Zheng, L.; Qin, L. Angiogenesis Assays for the Evaluation of Angiogenic Properties of Orthopaedic Biomaterials—A General Review. Adv. Healthc. Mater. 2017, 6, 1600434. [Google Scholar] [CrossRef]
- Irvin, M.W.; Zijlstra, A.; Wikswo, J.P.; Pozzi, A. Techniques and assays for the study of angiogenesis. Exp. Boil. Med. 2014, 239, 1476–1488. [Google Scholar] [CrossRef] [Green Version]










| Release | VEGF-Loaded | ||
|---|---|---|---|
| Days | 20 ng | 50 ng | 100 ng |
| Concentration (ng/mL) | Concentration (ng/mL) | Concentration (ng/mL) | |
| 1 | 18.5 ± 0.80 | 43.17 ± 3.00 | 84.26 ± 4.78 |
| 2 | 1.95 ± 0.06 | 6.12 ± 2.60 | 11.03 ± 0.99 |
| 3 | 0.23 ± 0.05 | 0.92 ± 0.53 | 1.82 ± 1.27 |
| 7 | 0.05 ± 0.00 | 0.10 ± 0.15 | 0.24 ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomino-Durand, C.; Lopez, M.; Marchandise, P.; Martel, B.; Blanchemain, N.; Chai, F. Chitosan/Polycyclodextrin (CHT/PCD)-Based Sponges Delivering VEGF to Enhance Angiogenesis for Bone Regeneration. Pharmaceutics 2020, 12, 784. https://doi.org/10.3390/pharmaceutics12090784
Palomino-Durand C, Lopez M, Marchandise P, Martel B, Blanchemain N, Chai F. Chitosan/Polycyclodextrin (CHT/PCD)-Based Sponges Delivering VEGF to Enhance Angiogenesis for Bone Regeneration. Pharmaceutics. 2020; 12(9):784. https://doi.org/10.3390/pharmaceutics12090784
Chicago/Turabian StylePalomino-Durand, Carla, Marco Lopez, Pierre Marchandise, Bernard Martel, Nicolas Blanchemain, and Feng Chai. 2020. "Chitosan/Polycyclodextrin (CHT/PCD)-Based Sponges Delivering VEGF to Enhance Angiogenesis for Bone Regeneration" Pharmaceutics 12, no. 9: 784. https://doi.org/10.3390/pharmaceutics12090784
APA StylePalomino-Durand, C., Lopez, M., Marchandise, P., Martel, B., Blanchemain, N., & Chai, F. (2020). Chitosan/Polycyclodextrin (CHT/PCD)-Based Sponges Delivering VEGF to Enhance Angiogenesis for Bone Regeneration. Pharmaceutics, 12(9), 784. https://doi.org/10.3390/pharmaceutics12090784