Development and Optimization of Naringenin-Loaded Chitosan-Coated Nanoemulsion for Topical Therapy in Wound Healing
Abstract
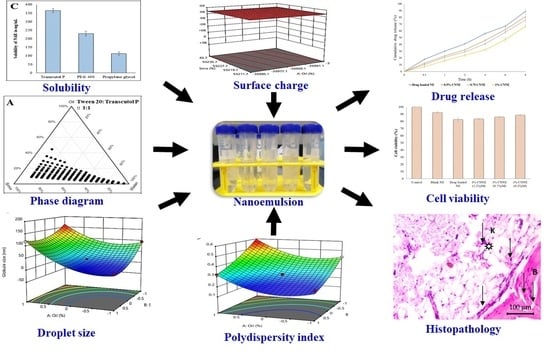
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Screening of Nanoemulsion Components
2.3. Selection of Surfactant and Co-Surfactant Ratio Using Pseudoternary Phase Diagrams
2.4. Preparation of Blank Nanoemulsion
2.5. Optimization of NRG Nanoemulsion Using Box–Behnken Statistical Design
× B2 + b33 × C2
2.6. Development of Drug-Loaded Nanoemulsion
2.7. Preparation of Chitosan-Coated Naringenin Nanoemulsion
2.8. Determination of Morphology of the Developed CNNE
2.9. Determination of the pH and Viscosity of the CNNEs
2.10. Ex Vivo Mucoadhesive Strength
2.11. In Vitro Release Study for NAR
2.12. In Vitro Cytotoxicity Study
2.13. Animal Facility
2.14. Abrasion Wound Healing Model
2.15. Histopathological Study
2.16. Statistical Analysis
3. Results and Discussion
3.1. Selection of Suitable Components for the Development of NAR Nanoemulsion
3.2. Pseudoternary Phase Diagrams in Determining Optimum Smix Ratio
3.3. Development of NE Formulation
3.4. Optimization of Nanoemulsion by Box–Behnken Statistical Design
3.4.1. Effect of Independent Variable on Globule Size
× C + 25.92 × B2 − 13.55 × B × C + 0.3408 × C2
3.4.2. Effect of Independent Variable on PDI
0.0012 × A × C + 0.0273 × B2 + 0.0115 × B × C + 0.0690 × C2
3.4.3. Effect of Independent Variables on Surface Charge
0.1000 × B × C + 0.9300 × A2 − 0.1200 × B2 − 0.0800 × C2
3.5. Development of NAR-Loaded NE Formulation
3.6. Determination of pH and Viscosity of the NE Formulations
3.7. Ex Vivo Mucoadhesive Strength
3.8. In Vitro Drug Release of NE
3.9. In Vitro Cytotoxicity Study
3.10. Construction of Wound Healing
3.11. Histopathological Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Croitoru, A.-M.; Ficai, D.; Ficai, A.; Mihailescu, N.; Andronescu, E.; Turculet, C.F. Nanostructured Fibers Containing Natural or Synthetic Bioactive Compounds in Wound Dressing Applications. Materials (Basel) 2020, 13, 2407. [Google Scholar] [CrossRef]
- Morsy, M.A.; Abdel-Latif, R.G.; Nair, A.B.; Venugopala, K.N.; Ahmed, A.F.; Elsewedy, H.S.; Shehata, T.M. Preparation and Evaluation of Atorvastatin-Loaded Nanoemulgel on Wound-Healing Efficacy. Pharmaceutics 2019, 11, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moroz, A.; Deffune, E. Platelet-rich plasma and chronic wounds: Remaining fibronectin may influence matrix remodeling and regeneration success. Cytotherapy 2013, 15, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Phillips, T.J. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605, quiz 605–586. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.; Abadir, P.; Brem, H.; Carter, M.; Conner-Kerr, T.; Davidson, J.; DiPietro, L.; Falanga, V.; Fife, C.; Gardner, S.; et al. Chronic wound repair and healing in older adults: Current status and future research. J. Am. Geriatr. Soc. 2015, 63, 427–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Cowin, A.J. New Innovations in Wound Healing and Repair. Int. J. Mol. Sci. 2019, 20, 1724. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, H.; Pandey, M.; Lim, Y.Q.; Low, C.Y.; Lee, C.T.; Marilyn, T.C.L.; Loh, H.S.; Lim, Y.P.; Lee, C.F.; Bhattamishra, S.K.; et al. Silver nanoparticles: Advanced and promising technology in diabetic wound therapy. Mater. Sci. Eng. C 2020, 112, 110925. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Dehkordi, A.N.; Babaheydari, F.M.; Chehelgerdi, M.; Dehkordi, S.R. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef] [Green Version]
- Sivamani, R.K.; Ma, B.R.; Wehrli, L.N.; Maverakis, E. Phytochemicals and Naturally Derived Substances for Wound Healing. Adv. Wound Care 2012, 1, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals (Basel) 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic Properties of Naringenin: A Citrus Fruit Polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Kotta, S.; Abdullah, S.T.; Fahmy, U.A.; Alfaleh, M.A.; Asfour, H.Z. Formulation Design, Statistical Optimization, and In Vitro Evaluation of a Naringenin Nanoemulsion to Enhance Apoptotic Activity in A549 Lung Cancer Cells. Pharmaceuticals (Basel) 2020, 13, 152. [Google Scholar] [CrossRef]
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic Acid Decorated Naringenin Nanoparticles: Appraisal of Chemopreventive and Curative Potential for Lung Cancer. Pharmaceutics 2018, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Jacob, S.; Nair, A.B. Cyclodextrin complexes: Perspective from drug delivery and formulation. Drug Dev. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef]
- Islam, N.; Dmour, I.; Taha, M.O. Degradability of chitosan micro/nanoparticles for pulmonary drug delivery. Heliyon 2019, 5, e01684. [Google Scholar] [CrossRef] [Green Version]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. (Indore) 2016, 4, 411–427. [Google Scholar]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Kumria, R.; Al-Dhubiab, B.E.; Shah, J.; Nair, A.B. Formulation and Evaluation of Chitosan-Based Buccal Bioadhesive Films of Zolmitriptan. J. Pharm. Innov. 2018, 13, 133–143. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, H.; Gorain, B.; Chatterjee, B.; Mandal, U.K.; Sengupta, P.; Tekade, R.K. Pharmacokinetic and Pharmacodynamic Features of Nanoemulsion Following Oral, Intravenous, Topical and Nasal Route. Curr. Pharm. Des. 2017, 23, 2504–2531. [Google Scholar] [CrossRef] [PubMed]
- Gorain, B.; Choudhury, H.; Nair, A.B.; Dubey, S.K.; Kesharwani, P. Theranostic application of nanoemulsions in chemotherapy. Drug Discov. Today 2020, 25, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Gorain, B.; Pandey, M.; Chatterjee, L.A.; Sengupta, P.; Das, A.; Molugulu, N.; Kesharwani, P. Recent Update on Nanoemulgel as Topical Drug Delivery System. J. Pharm. Sci. 2017, 106, 1736–1751. [Google Scholar] [CrossRef]
- Kumbhar, S.A.; Kokare, C.R.; Shrivastava, B.; Choudhury, H. Specific and Sensitive RP-HPLC Method Development and Validation for the Determination of Aripiprazole: Application in Preformulation Screening of Nanoemulsion. Cur. Nanomed. (Former. Recent Pat. Nanomed.) 2020, 10, 76–86. [Google Scholar] [CrossRef]
- Kumbhar, S.A.; Kokare, C.R.; Shrivastava, B.; Gorain, B.; Choudhury, H. Preparation, characterization, and optimization of asenapine maleate mucoadhesive nanoemulsion using Box-Behnken design: In vitro and in vivo studies for brain targeting. Int. J. Pharm. 2020, 586, 119499. [Google Scholar] [CrossRef]
- Choudhury, H.; Zakaria, N.F.B.; Tilang, P.A.B.; Tzeyung, A.S.; Pandey, M.; Chatterjee, B.; Alhakamy, N.A.; Bhattamishra, S.K.; Kesharwani, P.; Gorain, B.; et al. Formulation development and evaluation of rotigotine mucoadhesive nanoemulsion for intranasal delivery. J. Drug Deliv. Sci. Technol. 2019, 54, 101301. [Google Scholar] [CrossRef]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Development and evaluation of buccal films impregnated with selegiline-loaded nanospheres. Drug Deliv. 2016, 23, 2154–2162. [Google Scholar] [CrossRef] [Green Version]
- Gorain, B.; Choudhury, H.; Kundu, A.; Sarkar, L.; Karmakar, S.; Jaisankar, P.; Pal, T.K. Nanoemulsion strategy for olmesartan medoxomil improves oral absorption and extended antihypertensive activity in hypertensive rats. Colloids Interfaces B Biointerfaces 2014, 115, 286–294. [Google Scholar] [CrossRef]
- Shah, J.; Nair, A.B.; Jacob, S.; Patel, R.K.; Shah, H.; Shehata, T.M.; Morsy, M.A. Nanoemulsion Based Vehicle for Effective Ocular Delivery of Moxifloxacin Using Experimental Design and Pharmacokinetic Study in Rabbits. Pharmaceutics 2019, 11, 230. [Google Scholar] [CrossRef] [Green Version]
- Pathan, I.B.; Dwivedi, R.; Ambekar, W. Formulation and evaluation of ketoprofen loaded chitosan nanogel for pain management: Ex-vivo and In-vivo study. Ars Pharm. 2019, 60, 101–108. [Google Scholar]
- Nair, A.B.; Shah, J.; Al-Dhubiab, B.E.; Patel, S.S.; Morsy, M.A.; Patel, V.; Chavda, V.; Jacob, S.; Sreeharsha, N.; Shinu, P.; et al. Development of Asialoglycoprotein Receptor-Targeted Nanoparticles for Selective Delivery of Gemcitabine to Hepatocellular Carcinoma. Molecules (Basel) 2019, 24, 4566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendekal, M.S.; Tegginamat, P.K. Formulation and evaluation of a bioadhesive patch for buccal delivery of tizanidine. Acta Pharm. Sin. B 2012, 2, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Jacob, S.; Nair, A.B.; Al-Dhubiab, B.E. Preparation and evaluation of niosome gel containing acyclovir for enhanced dermal deposition. J. Liposome Res. 2017, 27, 283–292. [Google Scholar] [CrossRef]
- Raja, M.A.G.; Katas, H.; Hamid, Z.A. Penetration and silencing activity of VEGF dicer substrate siRNA vectorized by chitosan nanoparticles in monolayer culture and a solid tumor model in vitro for potential application in tumor therapy. J. Nanomater. 2016, 2016, 7201204. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.; Morsy, M.A.; Jacob, S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018, 79, 373–382. [Google Scholar] [CrossRef]
- Bektas, N.; Şenel, B.; Yenilmez, E.; Özatik, O.; Arslan, R. Evaluation of wound healing effect of chitosan-based gel formulation containing vitexin. Saudi Pharm. J. 2020, 28, 87–94. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of Micro- and Nanoemulsions as Vehicles for Essential Oils: Formulation, Preparation and Stability. Nanomaterials (Basel) 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion components screening and selection: A technical note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef]
- Shah, H.; Nair, A.B.; Shah, J.; Bharadia, P.; Al-Dhubiab, B.E. Proniosomal gel for transdermal delivery of lornoxicam: Optimization using factorial design and in vivo evaluation in rats. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2019, 27, 59–70. [Google Scholar] [CrossRef]
- Hidajat, M.J.; Jo, W.; Kim, H.; Noh, J. Effective Droplet Size Reduction and Excellent Stability of Limonene Nanoemulsion Formed by High-Pressure Homogenizer. Colloids Interfaces 2020, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, H.; Gorain, B.; Karmakar, S.; Biswas, E.; Dey, G.; Barik, R.; Mandal, M.; Pal, T.K. Improvement of cellular uptake, in vitro antitumor activity and sustained release profile with increased bioavailability from a nanoemulsion platform. Int. J. Pharm. 2014, 460, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Nair, A.B.; Shah, H.; Jacob, S.; Shehata, T.M.; Morsy, M.A. Enhancement in antinociceptive and anti-inflammatory effects of tramadol by transdermal proniosome gel. Asian J. Pharm. Sci. 2019. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Prasongsuk, S.; Sabliov, C.M. Effect of Surfactant Concentrations on Physicochemical Properties and Functionality of Curcumin Nanoemulsions under Conditions Relevant to Commercial Utilization. Molecules (Basel) 2019, 24, 2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadhave, D.; Gorain, B.; Tagalpallewar, A.; Kokare, C. Intranasal teriflunomide microemulsion: An improved chemotherapeutic approach in glioblastoma. J. Drug Deliv. Sci. Technol. 2019, 51, 276–289. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Yeun, O.C.; Yin, H.M.; Lynn, T.W.; Tine, C.L.Y.; Wi, N.S.; Yen, K.C.C.; Phing, C.S.; Kesharwani, P.; et al. Perspectives of Nanoemulsion Strategies in The Improvement of Oral, Parenteral and Transdermal Chemotherapy. Curr. Pharm. Biotechnol. 2018, 19, 276–292. [Google Scholar] [CrossRef]
- Ali, H.H.; Hussein, A.A. Oral nanoemulsions of candesartan cilexetil: Formulation, characterization and in vitro drug release studies. AAPS Open 2017, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Luesakul, U.; Puthong, S.; Sansanaphongpricha, K.; Muangsin, N. Quaternized chitosan-coated nanoemulsions: A novel platform for improving the stability, anti-inflammatory, anti-cancer and transdermal properties of Plai extract. Carbohydr. Polym. 2020, 230, 115625. [Google Scholar] [CrossRef]
- Kuo, S.-H.; Shen, C.-J.; Shen, C.-F.; Cheng, C.-M. Role of pH Value in Clinically Relevant Diagnosis. Diagnostics (Basel) 2020, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S.; Al-Dhubiab, B.; Attimarad, M.; Harsha, S. Basic considerations in the dermatokinetics of topical formulations. Braz. J. Pharm. Sci. 2013, 49, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Tan, Q.; Liu, W.; Guo, C.; Zhai, G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int. J. Nanomed. 2011, 6, 1621–1630. [Google Scholar] [CrossRef] [Green Version]
- Natesan, S.; Sugumaran, A.; Ponnusamy, C.; Thiagarajan, V.; Palanichamy, R.; Kandasamy, R. Chitosan stabilized camptothecin nanoemulsions: Development, evaluation and biodistribution in preclinical breast cancer animal mode. Int. J. Biol. Macromol. 2017, 104, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, R.M.; Abdel-Mohsen, A.M.; Hrdina, R.; Burgert, L.; Fohlerova, Z.; Pavliňák, D.; Sayed, O.N.; Jancar, J. Wound dressing based on chitosan/hyaluronan/nonwoven fabrics: Preparation, characterization and medical applications. Int. J. Biol. Macromol. 2016, 89, 725–736. [Google Scholar] [CrossRef]
- Kocyigit, A.; Koyuncu, I.; Dikilitas, M.; Bahadori, F.; Turkkan, B. Cytotoxic, genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines. Asian Pac. J. Trop. Biomed. 2016, 6, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.P.; Birundha, K.; Kaveri, K.; Devi, K.T.R. Antioxidant studies of chitosan nanoparticles containing naringenin and their cytotoxicity effects in lung cancer cells. Int. J. Biol. Macromol. 2015, 78, 87–95. [Google Scholar] [CrossRef]
- Dai, T.; Kharkwal, G.B.; Tanaka, M.; Huang, Y.Y.; de Arce, V.J.B.; Hamblin, M.R. Animal models of external traumatic wound infections. Virulence 2011, 2, 296–315. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Al-Roujayee, A.S. Naringenin improves the healing process of thermally-induced skin damage in rats. J. Int. Med. Res. 2017, 45, 570–582. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.M.; Al-Rawi, N.; Abbas, M.A.; Al-Khateeb, I. Naringenin potentiated β-sitosterol healing effect on the scratch wound assay. Res. Pharm. Sci. 2019, 14, 566–573. [Google Scholar] [CrossRef]












 : granulated tissue).
: granulated tissue).
 : granulated tissue).
: granulated tissue).
| Batch | Values of Independent Variables | Actual Responses | ||||
| A (% v/v) | B (% v/v) | C (% v/v) | Y1 (nm) | Y2 | Y3 (mV) | |
| 1 | 0 | −1 | 1 | 115.39 | 0.493 | −9.2 |
| 2 | 0 | 1 | 1 | 19.1 | 0.231 | −13.6 |
| 3 | 1 | −1 | 0 | 156 | 0.57 | −8.6 |
| 4 | 0 | 0 | 0 | 29.29 | 0.258 | −11.3 |
| 5 | 1 | 1 | 0 | 118.7 | 0.324 | −12.2 |
| 6 | −1 | −1 | 0 | 95.3 | 0.43 | −8.56 |
| 7 | −1 | 0 | −1 | 15.69 | 0.343 | −10.2 |
| 8 | 0 | 0 | 0 | 29.14 | 0.279 | −11.5 |
| 9 | 0 | −1 | −1 | 63.01 | 0.519 | −9.4 |
| 10 | 1 | 0 | 1 | 122.4 | 0.517 | −10.5 |
| 11 | 0 | 0 | 0 | 26.98 | 0.256 | −11.2 |
| 12 | 0 | 0 | 0 | 27.45 | 0.287 | −10.9 |
| 13 | 1 | 0 | −1 | 122.6 | 0.516 | −10.2 |
| 14 | 0 | 0 | 0 | 28.87 | 0.256 | −11.1 |
| 15 | 0 | 1 | −1 | 20.93 | 0.211 | −13.4 |
| 16 | −1 | 1 | 0 | 10.23 | 0.224 | −12.2 |
| 17 | −1 | 0 | 1 | 17.22 | 0.339 | −10.5 |
| Independent Variable | Levels | |||||
| Low (−1) | Medium (0) | High (+1) | ||||
| A = Oil (%v/v) | 5% | 10% | 15% | |||
| B = Smix (%v/v) | 35% | 40% | 45% | |||
| C = Water (%v/v) | 40% | 50% | 60% | |||
| Dependent variables | ||||||
| Y1 = Particle size | ||||||
| Y2 = Polydispersity index | ||||||
| Y3 = Zeta potential | ||||||
| Source | Sum of Squares | Degree of freedom (Df) | F-Ratio | p-Value |
|---|---|---|---|---|
| A: Oil | 18,169.90 | 1 | 223.31 | <0.0001 |
| B: Smix | 8498.17 | 1 | 104.44 | <0.0001 |
| C: Aqueous phase | 336.44 | 1 | 4.13 | 0.0815 |
| A2 | 7005.83 | 1 | 86.10 | <0.0001 |
| AB | 570.49 | 1 | 7.01 | 0.0330 |
| AC | 0.7482 | 1 | 0.0092 | 0.9263 |
| BC | 734.68 | 1 | 9.03 | 0.0198 |
| B2 | 2828.99 | 1 | 34.77 | 0.0006 |
| C2 | 0.4889 | 1 | 0.0060 | 0.9404 |
| Source | Sum of Squares | Df | F-Ratio | p-Value |
|---|---|---|---|---|
| A: Oil | 0.0437 | 1 | 73.67 | <0.0001 |
| B: Smix | 0.1306 | 1 | 220.30 | <0.0001 |
| C: Aqueous phase | 0.0000 | 1 | 0.0171 | 0.8997 |
| A2 | 0.0360 | 1 | 60.82 | 0.0001 |
| AB | 0.0004 | 1 | 0.6749 | 0.4384 |
| AC | 6.250 × 10−6 | 1 | 0.0105 | 0.9211 |
| BC | 0.0005 | 1 | 0.8926 | 0.3762 |
| B2 | 0.0031 | 1 | 5.29 | 0.0551 |
| C2 | 0.0201 | 1 | 33.85 | 0.0007 |
| Source | Sum of Squares | Df | F-Ratio | p-Value |
|---|---|---|---|---|
| A: Oil | 0.0002 | 1 | 0.0034 | 0.9552 |
| B: Smix | 30.58 | 1 | 517.74 | <0.0001 |
| C: Aqueous phase | 0.0450 | 1 | 0.7620 | 0.4117 |
| A2 | 3.64 | 1 | 61.66 | 0.0001 |
| AB | 0.0004 | 1 | 0.0068 | 0.9367 |
| AC | 0.0000 | 1 | 0.0000 | 1.0000 |
| BC | 0.0400 | 1 | 0.6773 | 0.4376 |
| B2 | 0.0606 | 1 | 1.03 | 0.3447 |
| C2 | 0.0269 | 1 | 0.4563 | 0.5210 |
| Formulation Details | Globule Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| NAR-loaded NE | 15.69 ± 2.32 | 0.330 | –8.33 ± 3.09 |
| 0.5% chitosan-coated naringenin nanoemulsion (CNNE) | 97.48 ± 6.23 | 0.245 | +29.5 ± 6.40 |
| 0.75% CNNE | 105.3 ± 6.56 | 0.222 | +33.8 ± 3.30 |
| 1% CNNE | 156 ± 7.34 | 0.419 | +44.4 ± 5.57 |
| Formulation Details | Measured pH | Measured Viscosity (cP) |
|---|---|---|
| NAR-loaded NE | 5.84 ± 0.060 | 54.66 ± 1.52 |
| 0.5% CNNE | 4.34 ± 0.060 | 169.3 ± 5.13 |
| 0.75% CNNE | 4.44 ± 0.045 | 335 ± 8.55 |
| 1% CNNE | 4.53 ± 0.150 | 658.3 ± 12.08 |
| Formulation | Zero-Order | First Order | Higuchi | Hixon–Crowell |
|---|---|---|---|---|
| Drug-loaded NE | 0.956 | 0.867 | 0.968 | 0.893 |
| 0.5% CNNE | 0.972 | 0.886 | 0.981 | 0.923 |
| 0.75% CNNE | 0.966 | 0.879 | 0.975 | 0.931 |
| 1.0% CNNE | 0.976 | 0.865 | 0.951 | 0.910 |
| Parameters | Control | Drug-Free Chitosan-Coated NE | CNNE (1%) | |||
|---|---|---|---|---|---|---|
| 0 Day | 14 Day | 0 Day | 14 Day | 0 Day | 14 Day | |
| Density of blood vessels | + | ++ | + | ++ | + | +++ |
| Thickness of epidermis | + | + | + | ++ | + | +++ |
| Number of inflammatory cells | +++ | ++ | +++ | ++ | +++ | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akrawi, S.H.; Gorain, B.; Nair, A.B.; Choudhury, H.; Pandey, M.; Shah, J.N.; Venugopala, K.N. Development and Optimization of Naringenin-Loaded Chitosan-Coated Nanoemulsion for Topical Therapy in Wound Healing. Pharmaceutics 2020, 12, 893. https://doi.org/10.3390/pharmaceutics12090893
Akrawi SH, Gorain B, Nair AB, Choudhury H, Pandey M, Shah JN, Venugopala KN. Development and Optimization of Naringenin-Loaded Chitosan-Coated Nanoemulsion for Topical Therapy in Wound Healing. Pharmaceutics. 2020; 12(9):893. https://doi.org/10.3390/pharmaceutics12090893
Chicago/Turabian StyleAkrawi, Sabah H., Bapi Gorain, Anroop B. Nair, Hira Choudhury, Manisha Pandey, Jigar N. Shah, and Katharigatta N. Venugopala. 2020. "Development and Optimization of Naringenin-Loaded Chitosan-Coated Nanoemulsion for Topical Therapy in Wound Healing" Pharmaceutics 12, no. 9: 893. https://doi.org/10.3390/pharmaceutics12090893
APA StyleAkrawi, S. H., Gorain, B., Nair, A. B., Choudhury, H., Pandey, M., Shah, J. N., & Venugopala, K. N. (2020). Development and Optimization of Naringenin-Loaded Chitosan-Coated Nanoemulsion for Topical Therapy in Wound Healing. Pharmaceutics, 12(9), 893. https://doi.org/10.3390/pharmaceutics12090893