Investigation of the Transport Pathways Associated with Enhanced Brain Delivery of Peptide Drugs by Intranasal Coadministration with Penetratin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Examination of Systemic Absorption and Brain Distribution of Peptide Drugs after Intranasal and Subcutaneous Administration
2.3.1. Preparation of the Exendin-4 and L-penetratin Solutions
2.3.2. Intranasal and Subcutaneous Administration Study
2.4. Examination of Brain and Trigeminal Nerve Distribution after Intranasal Administration of Cy7-Labeled Peptide Drugs
2.4.1. Labeling Exendin-4 and Insulin with the Cy7 Fluorescent Dye
2.4.2. Ex Vivo Brain and Trigeminal Nerve Imaging after Intranasal Administration
2.5. Histoimmunological Staining of Brain Specimens after Intranasal Administration of Peptide Drugs
2.6. Statistical Analysis
3. Results
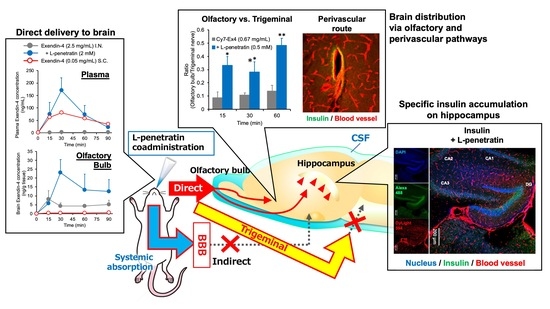
3.1. Contribution of Direct Nose-to-Brain Transport to the L-Penetratin-Enhanced Brain Delivery of Exendin-4
3.2. Comparison of the Contribution of Olfactory Mucosal Transport and Trigeminal Axonal Transport on L-Penetratin-Enhanced Peptide Brain Delivery
3.3. Detailed Evaluation of the Distribution of Peptide Drugs Delivered to the Brain by Coadministration with L-Penetratin
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Samaridou, E.; Alonso, M. Nose-to-brain peptide delivery–The potential of nanotechnology. Bioorganic Med. Chem. 2018, 26, 2888–2905. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H., 2nd. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef]
- Inoue, D.; Furubayashi, T.; Tanaka, A.; Sakane, T.; Sugano, K. Effect of Cerebrospinal Fluid Circulation on Nose-to-Brain Direct Delivery and Distribution of Caffeine in Rats. Mol. Pharm. 2020, 17, 4067–4076. [Google Scholar] [CrossRef]
- Iwasaki, S.; Yamamoto, S.; Sano, N.; Tohyama, K.; Kosugi, Y.; Furuta, A.; Hamada, T.; Igari, T.; Fujioka, Y.; Hirabayashi, H.; et al. Direct Drug Delivery of Low-Permeable Compounds to the Central Nervous System Via Intranasal Administration in Rats and Monkeys. Pharm. Res. 2019, 36, 76. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, H.M.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Kamei, N.; Takeda-Morishita, M. Brain delivery of insulin boosted by intranasal coadministration with cell-penetrating peptides. J. Control. Release 2015, 197, 105–110. [Google Scholar] [CrossRef]
- Yadav, S.; Gattacceca, F.; Panicucci, R.; Amiji, M.M. Comparative Biodistribution and Pharmacokinetic Analysis of Cyclosporine-A in the Brain upon Intranasal or Intravenous Administration in an Oil-in-Water Nanoemulsion Formulation. Mol. Pharm. 2015, 12, 1523–1533. [Google Scholar] [CrossRef]
- Nedelcovych, M.T.; Gadiano, A.J.; Wu, Y.; Manning, A.A.; Thomas, A.G.; Khuder, S.S.; Yoo, S.W.; Xu, J.; McArthur, J.C.; Haughey, N.J.; et al. Pharmacokinetics of Intranasal versus Subcutaneous Insulin in the Mouse. ACS Chem. Neurosci. 2018, 9, 809–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamei, N.; Shingaki, T.; Kanayama, Y.; Tanaka, M.; Zochi, R.; Hasegawa, K.; Watanabe, Y.; Takeda-Morishita, M. Visualization and Quantitative Assessment of the Brain Distribution of Insulin through Nose-to-Brain Delivery Based on the Cell-Penetrating Peptide Noncovalent Strategy. Mol. Pharm. 2016, 13, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Tanaka, M.; Choi, H.; Okada, N.; Ikeda, T.; Itokazu, R.; Takeda-Morishita, M. Effect of an Enhanced Nose-to-Brain Delivery of Insulin on Mild and Progressive Memory Loss in the Senescence-Accelerated Mouse. Mol. Pharm. 2017, 14, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Okada, N.; Ikeda, T.; Choi, H.; Fujiwara, Y.; Okumura, H.; Takeda-Morishita, M. Effective nose-to-brain delivery of exendin-4 via coadministration with cell-penetrating peptides for improving progressive cognitive dysfunction. Sci. Rep. 2018, 8, 17641. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, E.S.; Kamei, N.; Fujiwara, Y.; Okumura, H.; Yuasa, T.; Kato, M.; Arime, K.; Nonomura, A.; Ogino, H.; Hirano, S.; et al. Systemic and brain delivery of leptin via intranasal coadministration with cell-penetrating peptides and its therapeutic potential for obesity. J. Control. Release 2020, 319, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Yamaoka, A.; Fukuyama, Y.; Itokazu, R.; Takeda-Morishita, M. Noncovalent Strategy with Cell-Penetrating Peptides to Facilitate the Brain Delivery of Insulin through the Blood-Brain Barrier. Biol. Pharm. Bull. 2018, 41, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirai, S.; Yashiki, T.; Matsuzawa, T.; Mima, H. Absorption of drugs from the nasal mucosa of rat. Int. J. Pharm. 1981, 7, 317–325. [Google Scholar] [CrossRef]
- Johnson, N.J.; Hanson, L.R.; Frey, W.H., 2nd. Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures. Mol. Pharm. 2010, 7, 884–893. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, T.; Kaneko, M.; Niide, T.; Akiyama, F.; Kakizaki, S.; Ibaraki, H.; Shiraishi, S.; Takashima, Y.; Suzuki, T.; Seta, Y. Enhancement of nose-to-brain delivery of hydrophilic macromolecules with stearate- or polyethylene glycol-modified arginine-rich peptide. Int. J. Pharm. 2017, 530, 195–200. [Google Scholar] [CrossRef]
- Kamei, N.; Morishita, M.; Takayama, K. Importance of intermolecular interaction on the improvement of intestinal therapeutic peptide/protein absorption using cell-penetrating peptides. J. Control. Release 2009, 136, 179–186. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Wolak, D.J.; Pizzo, M.E.; Thorne, R.G. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J. Cereb. Blood Flow Metab. 2015, 35, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [Green Version]
- Pizzo, M.E.; Wolak, D.J.; Kumar, N.N.; Brunette, E.; Brunnquell, C.L.; Hannocks, M.J.; Abbott, N.J.; Meyerand, M.E.; Sorokin, L.; Stanimirovic, D.B.; et al. Intrathecal antibody distribution in the rat brain: Surface diffusion, perivascular transport and osmotic enhancement of delivery. J. Physiol. 2018, 596, 445–475. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.N.; Lochhead, J.J.; Pizzo, M.E.; Nehra, G.; Boroumand, S.; Greene, G.; Thorne, R.G. Delivery of immunoglobulin G antibodies to the rat nervous system following intranasal administration: Distribution, dose-response, and mechanisms of delivery. J. Control. Release 2018, 286, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Bourganis, V.; Kammona, O.; Alexopoulos, A.; Kiparissides, C. Recent Advances in Carrier Mediated Nose-to-Brain Delivery of Pharmaceutics. Eur. J. Pharm. Biopharm. 2018, 128, 337–362. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Perets, N.; Betzer, O.; Ben-Shaul, S.; Sheinin, A.; Michaelevski, I.; Popovtzer, R.; Offen, D.; Levenberg, S. Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog siRNA Repairs Complete Spinal Cord Injury. ACS Nano 2019, 13, 10015–10028. [Google Scholar] [CrossRef] [PubMed]
- Meredith, E.M.; Salameh, T.S.; Banks, W.A. Intranasal Delivery of Proteins and Peptides in the Treatment of Neurodegenerative Diseases. AAPS J. 2015, 17, 780–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Banks, W.A.; During, M.J.; Niehoff, M.L. Brain uptake of the glucagon-like peptide-1 antagonist exendin(9-39) after intranasal administration. J. Pharmacol. Exp. Ther. 2004, 309, 469–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brüning, J.C.; Gautam, D.; Burks, D.J.; Gillette, J.; Schubert, M.; Orban, P.C.; Klein, R.; Krone, W.; Müller-Wieland, D.; Kahn, C.R. Role of brain insulin receptor in control of body weight and reproduction. Science 2000, 289, 2122–2125. [Google Scholar] [CrossRef] [PubMed]
- Kleinridders, A.; Ferris, H.A.; Cai, W.; Kahn, C.R. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 2014, 63, 2232–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamei, N.; Suwabe, S.; Arime, K.; Bando, H.; Murata, K.; Yamaguchi, M.; Yokoyama, N.; Tanaka, E.; Hashimoto, A.; Kanazawa, T.; et al. Investigation of the Transport Pathways Associated with Enhanced Brain Delivery of Peptide Drugs by Intranasal Coadministration with Penetratin. Pharmaceutics 2021, 13, 1745. https://doi.org/10.3390/pharmaceutics13111745
Kamei N, Suwabe S, Arime K, Bando H, Murata K, Yamaguchi M, Yokoyama N, Tanaka E, Hashimoto A, Kanazawa T, et al. Investigation of the Transport Pathways Associated with Enhanced Brain Delivery of Peptide Drugs by Intranasal Coadministration with Penetratin. Pharmaceutics. 2021; 13(11):1745. https://doi.org/10.3390/pharmaceutics13111745
Chicago/Turabian StyleKamei, Noriyasu, Susumu Suwabe, Kenji Arime, Hidemi Bando, Kaho Murata, Maika Yamaguchi, Natsuki Yokoyama, Erina Tanaka, Ayaka Hashimoto, Takanori Kanazawa, and et al. 2021. "Investigation of the Transport Pathways Associated with Enhanced Brain Delivery of Peptide Drugs by Intranasal Coadministration with Penetratin" Pharmaceutics 13, no. 11: 1745. https://doi.org/10.3390/pharmaceutics13111745
APA StyleKamei, N., Suwabe, S., Arime, K., Bando, H., Murata, K., Yamaguchi, M., Yokoyama, N., Tanaka, E., Hashimoto, A., Kanazawa, T., Ago, Y., & Takeda-Morishita, M. (2021). Investigation of the Transport Pathways Associated with Enhanced Brain Delivery of Peptide Drugs by Intranasal Coadministration with Penetratin. Pharmaceutics, 13(11), 1745. https://doi.org/10.3390/pharmaceutics13111745