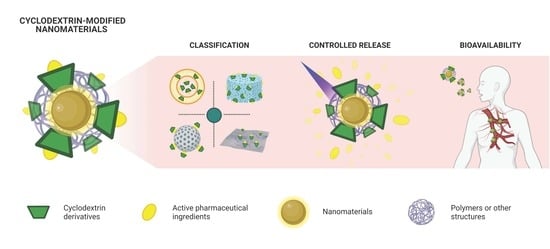
Cyclodextrin-Modified Nanomaterials for Drug Delivery: Classification and Advances in Controlled Release and Bioavailability
Abstract
:1. Introduction
2. Classification of Cyclodextrin-Modified Nanomaterials
2.1. Lipid-Based Nanocarriers
2.1.1. Liposomes
2.1.2. Nanoemulsions
2.1.3. Solid-Lipid Nanoparticles and Nanostructured Lipid Carriers
2.1.4. Lipid Micelles
2.2. Polymeric Nanocarriers
2.2.1. Natural Polymer-Based
2.2.2. Synthetic Polymer-Based
| System | CD | API | Loading Capacity | Loading Efficiency | Drug Release Mechanism | In Vivo Studies | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Lipid-based nanocarriers derivates | CD–drug loaded liposomes | Amino-deoxy-βCD | Pin1 inhibitor | - | 91% | Slow diffusive release | Pharmacokinetics, biodistribution, and efficacy | [55] |
| Double-loaded liposomes | Dimethyl-βCD | Paclitaxel | 1.2 mg/mL | 93% | Slow diffusive release | Pharmacokinetics and acute toxicity after intravenous administration | [57] | |
| Single and double-loaded deformable liposomes | HPβCD, SBEβCD and MEβCD | Butamben | 0.01% | 92–100% | Diffusive release | Ex vivo permeation and in vivo anesthetic effect | [58] | |
| Nanoemulsion with HPβCD and Tween-80 | HPβCD | Cinnamon essential oil | - | - | Slow diffusive release | - | [62] | |
| Multiple nanoemulsion (w/o/w) with HPβCD and poloxamer 188 | HPβCD | Pemetrexed | - | 95% | Quick diffusive release | Oral bioavailability and in vivo tumor growth inhibition effect | [63] | |
| SLN capped with βCD polymers | βCD | Benzophenone | 9–12% | 72–96% | Higuchi and Korsmeyer–Peppas kinetics | - | [67] | |
| NLC loaded with CD–drug complex | HPβCD and SBEβCD | Hydrochlorothiazide | 2–4% | 40–88% | Quick diffusive release | Diuretic activity after oral administration | [69] | |
| NLC loaded with CD–drug complex | HPβCD | Thymol | 2.2% | 79% | Higuchi kinetic | Ex vivo skin permeation | [74] | |
| Micelles assembled from HPβCD and glyceryl monostearate | HPβCD | Astaxanthin | 2.7% | 100% | pH change | Oral bioavailability, tissue distribution | [75] | |
| Polymeric nanocarriers | CD-cellulose nanocrystals | Glycidyltrimethyl ammonium chloride-βCD | Curcumin | 91 mg/g | 9% | More likely, cell internalization due to endocytosis followed by release into lysosomes | Bioavailability, in vivo nervous function | [78,79] |
| CD–drug inclusion complex loaded chitosan nanoparticles | Dimethyl-βCD | Salazosulfapyridine | 3–10% | 80–90% | Degradation of polymeric matrix | - | [82] | |
| Red blood membrane-coated nanogels formulated | HPβCD acrylate | Paclitaxel and IL-2 | 93% (500 µg Paclitaxel) | 32% (500 µg Paclitaxel) | pH change | Drug release in tumor microenvironment, bioavailability, biodistribution, antitumor efficacy, immune response | [83] | |
| Polysaccharide-based noncovalent assembly for targeted drug delivery | Permethyl-β-CD | Porphyrin modified paclitaxel | 31% | 85% | Enzyme-triggered drug release | - | [85] | |
| Nanoformulation based on PEGylated liposomal and nanocurcumin | HPβCD (+citric acid) | DOX + Curcumin | - | >95% (data not shown) | - | - | [88] | |
| Ocular nanosuspension based on commercial polymers | Methyl-βCD/HPβCD | Econazole Nitrate | 43–52% | - | Degradation of polymeric matrix | Ocular irritation, Bioavailability in tears | [89] | |
| Amino-βCD-containing polymers nanoassemblies | Amino-βCD with various alkyl chains | Ferulic acid | 4% | - | pH change | Biodistribution | [90] | |
| Dual stimuli-responsive supramolecular self-assemblies | βCD-graft-poly(2-(dimethylamino)ethyl methacrylate) | DOX | 13% | 66% | pH change and UV irradiation responsive release | - | [91] | |
| Multifunctional nanoconjugates | βCD-Maleic anhydride | Curcumin and DOX | 0.45 g/g and 0.32 g/g | 88% | pH change and temperature change | Blood markers, gene expression in liver tissue | [92] |
2.3. Polymeric Nanosystems Based on Cyclodextrins
2.4. Graphene Derivatives
2.5. Inorganic Nanoparticles
2.5.1. Mesoporous Silica Nanoparticles
2.5.2. Plasmonic Nanoparticles
2.5.3. Magnetic Nanoparticles
2.5.4. Quantum Dots
2.6. Other Nanosystems
2.6.1. As Nanovalves
2.6.2. Metal–Organic Frameworks
2.6.3. Janus Nanoparticles
2.6.4. Nanofibers
| System | CD | API | Loading Capacity | Loading Efficiency | Drug Release Mechanism | In Vivo Studies | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Polymeric nanosystems based on CDs | βCD polymer-based nanosponge | βCD | Temoporfin | - | - | Diffusive release under tumor spheroid conditions | - | [97] |
| βCD polymer-based nanocarrier | βCD | Sorafenib | ~5.7 and ~9.9 mg/g | - | Diffusive release under cell and mice pshysiological conditions | Toxicity and accumulation | [98] | |
| βCD-based polymer with RGD peptides | βCD | DOX | - | - | Diffusive release under carcinogenic cellular conditions | - | [99] | |
| Cationic βCD polymer with fluorescent probe nanocarrier | βCD | Diclofenac | 16% | 100% | Diffusive release under physiological conditions | - | [100] | |
| βCD polymer-based nanoparticle | βCD | Ethionamide and BDM-smart420-booster | ~21 and ~6 mg/g | - | - | - | [102] | |
| 25 mg/g | - | Diffusive release in vivo | Efficacy | [101] | ||||
| Pseudopolyrotaxane-βCD-based polymer | βCD | Novobiocin | 23% | - | Diffusive release under physiological conditions | - | [103] | |
| Vancomycin | 6% | - | ||||||
| Novobiocin | 18% | - | ||||||
| Vancomycin | 6% | - | ||||||
| βCD-based nanosponge tablet | βCD | Paracetamol, aceclofenac and caffeine | - | 81–89% | Diffusive release from the tablet | - | [104] | |
| βCD-based nanosponge suspension | βCD | Nifedipine | - | 78% | Diffusive release under simulated gastric fluid | Oral bioavailability | [105] | |
| βCD polymer-based nanosponge | βCD | Atorvastatin | - | 34% | Diffusive release in dialysis sac methods | Oral bioavailability, pharmacodynamics, and efficacy | [106] | |
| βCD-based nanosponge tablet | βCD | Febuxostat | - | 88–100% | Diffusive release using dissolution apparatus | Oral bioavailability | [107] | |
| βCD polymer-based nanosponge functionalized with gold nanoparticles | βCD | Phenylethylamine | 90% | - | - | - | [110] | |
| 2-amino-4-(4-chlorophenyl)-thiazole | 150% | - | ||||||
| Graphene derivatives | Multi-walled carbon nanotubes CD-Maleic Anhydride-N-Isopropylacrylamide-Fluorescein-folic acid | βCD | Curcumin + DOX | 29 wt% (curcumin) and 19 wt% (DOX) | 92% | Temperature change, pH change and laser irradiation at 808 nm | Progression and regression of tumor in BALB/c mice model | [116] |
| Graphene oxide-L-phenylalanine-βCD | βCD | DOX | 85%, | 79% | pH change | - | [118] | |
| Graphene oxide-Fe3O4-βCD | Mono-6-deoxy-6-ethylenediamino-βCD | DOX | 4% | 37% | pH change | - | [119] | |
| Methotrexate | 2% | 23% | ||||||
| Graphene oxide-βCD-poly(amido amine) dendrimer | aminated-βCD | DOX | 0.4 mg/mg | - | pH change | - | [120] | |
| Camptothecin | 4.0 mg/mg | |||||||
| Protoporphyrin IX | 0.8 mg/mg | |||||||
| Graphene oxide + mPEG−QPDMAEMA/α-CD supramolecular hydrogel | αCD | 5-fluorouracil | 4.6 mg/g | - | Temperature change, pH change and UV irradiation responsive release | - | [121] | |
| βCD/Ni nanoparticle-modified GO and mitochondrial ion-targeting peptide-grafted hyaluronic acid | Mono-6-deoxy-6-ethylenediamino-βCD | DOX | >36% | - | AMF responsive release | - | [122] | |
| Core: Curcumin@CD-oxide graphene, Shell: Gallic acid@Chitosan | 6-O-monotosyl-βCD | Curcumin | pH change | - | [123] | |||
| Gallic acid | ||||||||
| βCD/Carbon dots | βCD | DOX | 27% | - | pH change | - | [125] | |
| Associated to inorganic nanoparticles | Tetra-ortho-methoxy-substituted azobenzene/βCD-modified mesoporous silica nanoparticles | aminated-βCD | p-Coumalic acid | - | - | Green light (520 nm) | - | [128] |
| Gold nanostar modified with cationic βCD-based polymer | βCD | Phenylethylamine and piperine | 95% | 91–76% | - | - | [143] | |
| Electrospun CD/Ag nanoparticles nanofibers | HPβCD | Ag nanoparticles | - | - | ions on agar plates | - | [144] | |
| Fe3O4 magnetic nanoparticles functionalized with loaded mono-6-thio-βCD | 6-thio-βCD | DOX | 90% | - | pH change | - | [41] | |
| Nickel ferrite nanoparticles covered with CD-dextran polymers | Mono-6-deoxy-6-aminoethylamino-βCD | camptothecin | - | - | pH change | - | [146] | |
| ZnSe/ZnS quantum dots on βCD/chitosan polymer | βCD | Suberoylanilide hydroxamic acid | 22% | - | pH change | Biodistribution in a melanoma animal model injected subcutaneously | [148] | |
| Other nanosystems | Mesoporous silica nanoparticles modified with CDs/2-diazo-1,2-naphthoquinone nanovalves | βCD | DOX | 5% | 69% | NIR light irradiation | Intratumoral injection in tumor-bearing mice | [150] |
| MOF nanoparticles functionalized with iron (III) polycarboxylates/CDs | Phosphated CD | Azidothymidine-triphosphate | 8% | - | [154] | |||
| Janus gold nanostar–mesoporous silica nanoparticle modified with a thiolated photolabile molecule and proton-responsive benzimidazole-βCD | β-CD | DOX | - | - | NIR light irradiation | - | [156] | |
| βCD functionalized polyurethane fibrous membranes | βCD | Gentamicin sulphate | 68% | - | Diffusive release in PBS at pH 7.4 | Antibacterial activity against Gram positive Staphylococcus aureus and Gram negative Escherichia Coli | [157] |
3. Cyclodextrin Derivates for Stimuli-Controlled Drug Release
4. Bioavailability of Cyclodextrin-Modified Nanomaterials
5. Final Remarks
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Real, D.; Hoffmann, S.; Leonardi, D.; Salomon, C.; Goycoolea, F.M. Chitosan-based nanodelivery systems applied to the development of novel triclabendazole formulations. PLoS ONE 2018, 13, e0207625. [Google Scholar] [CrossRef]
- Sierpe, R.; Lang, E.; Jara, P.; Guerrero, A.R.; Chornik, B.; Kogan, M.J.; Yutronic, N. Gold Nanoparticles Interacting with β-Cyclodextrin-Phenylethylamine Inclusion Complex: A Ternary System for Photothermal Drug Release. ACS Appl. Mater. Interfaces 2015, 7, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Asela, I.; Noyong, M.; Simon, U.; Andrades-Lagos, J.; Campanini-Salinas, J.; Vásquez-Velásquez, D.; Kogan, M.; Yutronic, N.; Sierpe, R. Gold nanoparticles stabilized with βcyclodextrin-2-amino-4-(4-chlorophenyl) thiazole complex: A novel system for drug transport. PLoS ONE 2017, 12, e0185652. [Google Scholar] [CrossRef] [Green Version]
- Sierpe, R.; Noyong, M.; Simon, U.; Aguayo, D.; Huerta, J.; Kogan, M.J.; Yutronic, N. Construction of 6-thioguanine and 6-mercaptopurine carriers based on βcyclodextrins and gold nanoparticles. Carbohydr. Polym. 2017, 177, 22–31. [Google Scholar] [CrossRef]
- Adeoye, O.; Cabral-Marques, H. Cyclodextrin nanosystems in oral drug delivery: A mini review. Int. J. Pharm. 2017, 531, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Uyar, T. Electrospinning of cyclodextrin functional nanofibers for drug delivery applications. Pharmaceutics 2019, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Liu, W.; Huang, J.; Qiu, S.; Zhong, H.; Liu, D.; Liu, J. Cyclodextrin-based metal-organic frameworks (CD-MOFs) in pharmaceutics and biomedicine. Pharmaceutics 2018, 10, 271. [Google Scholar] [CrossRef] [Green Version]
- Aiassa, V.; Garnero, C.; Longhi, M.R.; Zoppi, A. Cyclodextrin multicomponent complexes: Pharmaceutical applications. Pharmaceutics 2021, 13, 1099. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 76, 1825–1845. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Past, present, and future of cyclodextrin research. Pure Appl. Chem. 2004, 76, 1825–1845. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Muankaew, C.; Loftsson, T. Cyclodextrin-Based Formulations: A Non-Invasive Platform for Targeted Drug Delivery. Basic Clin. Pharmacol. Toxicol. 2018, 122, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Real, D.; Leonardi, D.; Williams, R.O.; Repka, M.A.; Salomon, C.J. Solving the Delivery Problems of Triclabendazole Using Cyclodextrins. AAPS PharmSciTech 2018, 19, 2311–2321. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Priotti, J.; Codina, A.V.; Vasconi, M.D.; Quiroga, A.D.; Hinrichsen, L.I.; Leonardi, D.; Lamas, M.C. Synthesis and characterization of a new cyclodextrin derivative with improved properties to design oral dosage forms. Drug Deliv. Transl. Res. 2019, 9, 273–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. An Off. J. Am. Assoc. Pharm. Sci. 1995, 12, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Lv, P.; Zhou, C.; Zhao, Y.; Liao, X.; Yang, B. Cyclodextrin-based delivery systems for cancer treatment. Mater. Sci. Eng. C 2019, 96, 872–886. [Google Scholar] [CrossRef]
- Rodrigues, L.N.C.; Tavares, A.C.M.; Ferreira, B.T.; Reis, A.K.C.A.; Katiki, L.M. Inclusion complexes and self-assembled cyclodextrin aggregates for increasing the solubility of benzimidazoles. Braz. J. Pharm. Sci. 2019, 55. [Google Scholar] [CrossRef] [Green Version]
- Asim, M.H.; Nazir, I.; Jalil, A.; Laffleur, F.; Matuszczak, B.; Bernkop-Schnürch, A. Per-6-Thiolated Cyclodextrins: A Novel Type of Permeation Enhancing Excipients for BCS Class IV Drugs. ACS Appl. Mater. Interfaces 2020, 12, 7942–7950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F. Cyclodextrins in drug delivery systems and their effects on biological barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.R.; Vavia, P.R. Preparation and evaluation of taste masked famotidine formulation using drug/β-cyclodextrin/polymer ternary complexation approach. AAPS PharmSciTech 2008, 9, 544–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arima, H.; Higashi, T.; Motoyama, K. Improvement of the bitter taste of drugs by complexation with cyclodextrins: Applications, evaluations and mechanisms. Ther. Deliv. 2012, 3, 633–644. [Google Scholar] [CrossRef]
- Liu, T.; Wan, X.; Luo, Z.; Liu, C.; Quan, P.; Cun, D.; Fang, L. A donepezil/cyclodextrin complexation orodispersible film: Effect of cyclodextrin on taste-masking based on dynamic process and in vivo drug absorption. Asian J. Pharm. Sci. 2019, 14, 183–192. [Google Scholar] [CrossRef]
- Kant, A.; Linforth, R.S.T.; Hort, J.; Taylor, A.J. Effect of β-Cyclodextrin on Aroma Release and Flavor Perception. J. Agric. Food Chem. 2004, 52, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Popielec, A.; Loftsson, T. Effects of cyclodextrins on the chemical stability of drugs. Int. J. Pharm. 2017, 531, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Saraf, S.; Saraf, S. Cyclodextrin based novel drug delivery systems. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 23–42. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Mura, P. Advantages of the combined use of cyclodextrins and nanocarriers in drug delivery: A review. Int. J. Pharm. 2020, 579, 119181. [Google Scholar] [CrossRef]
- Liao, R.; Lv, P.; Wang, Q.; Zheng, J.; Feng, B.; Yang, B. Cyclodextrin-based biological stimuli-responsive carriers for smart and precision medicine. Biomater. Sci. 2017, 5, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, Y.; Liu, J. Smart stimuli-responsive drug delivery systems based on cyclodextrin: A review. Carbohydr. Polym. 2021, 251, 116871. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Rincón-López, J.; Almanza-Arjona, Y.C.; Riascos, A.P.; Rojas-Aguirre, Y. Technological evolution of cyclodextrins in the pharmaceutical field. J. Drug Deliv. Sci. Technol. 2021, 61, 102156. [Google Scholar] [CrossRef] [PubMed]
- Otero-Espinar, F.J.; Torres-Labandeira, J.J.; Alvarez-Lorenzo, C.; Blanco-Méndez, J. Cyclodextrins in drug delivery systems. J. Drug Deliv. Sci. Technol. 2010, 20, 289–301. [Google Scholar] [CrossRef]
- Novio, F. Design of targeted nanostructured coordination polymers (NCPS) for cancer therapy. Molecules 2020, 25, 3449. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, K.; Kogan, M.J.; Araya, E. Capping gold nanoparticles with albumin to improve their biomedical properties. Int. J. Nanomed. 2019, 14, 6387–6406. [Google Scholar] [CrossRef] [Green Version]
- Pham, S.H.; Choi, Y.; Choi, J. Stimuli-responsive nanomaterials for application in antitumor therapy and drug delivery. Pharmaceutics 2020, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Rahme, K.; He, Y.; Li, L.L.; Holmes, J.D.; O’Driscoll, C.M. Gold nanoparticles enlighten the future of cancer theranostics. Int. J. Nanomed. 2017, 12, 6131–6152. [Google Scholar] [CrossRef] [Green Version]
- Mrówczyński, R.; Jędrzak, A.; Szutkowski, K.; Grześkowiak, B.F.; Coy, E.; Markiewicz, R.; Jesionowski, T.; Jurga, S. Cyclodextrin-based magnetic nanoparticles for cancer therapy. Nanomaterials 2018, 8, 170. [Google Scholar] [CrossRef] [Green Version]
- Bolaños, K.; Sánchez-Navarro, M.; Tapia-Arellano, A.; Giralt, E.; Kogan, M.J.; Araya, E. Oligoarginine peptide conjugated to bsa improves cell penetration of gold nanorods and nanoprisms for biomedical applications. Pharmaceutics 2021, 13, 1204. [Google Scholar] [CrossRef]
- Liu, H.; Taylor, L.S.; Edgar, K.J. The role of polymers in oral bioavailability enhancement; A review. Polymer 2015, 77, 399–415. [Google Scholar] [CrossRef] [Green Version]
- Madkour, L.H. Nanoparticle and polymeric nanoparticle-based targeted drug delivery systems. In Nucleic Acids as Gene Anticancer Drug Delivery Therapy; Academic Press: Cambridge, MA, USA, 2019; pp. 191–240. ISBN 978-0-12-819777-6. [Google Scholar]
- Feczkó, T. Polymeric nanotherapeutics acting at special regions of body. J. Drug Deliv. Sci. Technol. 2021, 64, 102597. [Google Scholar] [CrossRef]
- Vetterlein, C.; Vásquez, R.; Bolaños, K.; Acosta, G.A.; Guzman, F.; Albericio, F.; Celis, F.; Campos, M.; Kogan, M.J.; Araya, E. Exploring the influence of Diels–Alder linker length on photothermal molecule release from gold nanorods. Colloids Surf. B Biointerfaces 2018, 166, 323–329. [Google Scholar] [CrossRef]
- Inostroza-Riquelme, M.; Vivanco, A.; Lara, P.; Guerrero, S.; Salas-Huenuleo, E.; Chamorro, A.; Leyton, L.; Bola os, K.; Araya, E.; Quest, A.F.G.; et al. Encapsulation of gold nanostructures and oil-in-water nanocarriers in microgels with biomedical potential. Molecules 2018, 23, 1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, S.; Shende, P. Cyclodextrins-modified metallic nanoparticles for effective cancer therapy. J. Control. Release 2021, 339, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Valetti, S.; Mura, S.; Stella, B.; Couvreur, P. Rational design for multifunctional non-liposomal lipid-based nanocarriers for cancer management: Theory to practice. J. Nanobiotechnol. 2013, 11 (Suppl. 1), 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinaud, M.C.; Real, D.; Fraga, C.M.; Lima, N.F.; De Souza Lino, R., Jr.; Leonardi, D.; Salomon, C.J. Nanodelivery of nitazoxanide: Impact on the metabolism of Taenia crassiceps cysticerci intracranially inoculated in mice. Ther. Deliv. 2020, 11, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Meng, M.H.W.; Zhao, H.; Iqbal, J.; Dai, R.; Deng, Y.; Lv, F. Luteolin-loaded solid lipid nanoparticles synthesis, characterization, & improvement of bioavailability, pharmacokinetics in vitro and vivo studies. J. Nanopart. Res. 2014, 16, 2347. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [PubMed]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.; Iqbal, J.; Awan, U.; Xin, N.; Dang, H.; Waryani, B.; Saeed, Y.; Ullah, K.; Dai, R.; Deng, Y. LX loaded nanoliposomes synthesis, characterization and cellular uptake studies in H2O2 stressed SH-SY5Y cells. J. Nanosci. Nanotechnol. 2014, 14, 4066–4071. [Google Scholar] [CrossRef] [PubMed]
- Russo Spena, C.; De Stefano, L.; Palazzolo, S.; Salis, B.; Granchi, C.; Minutolo, F.; Tuccinardi, T.; Fratamico, R.; Crotti, S.; D’Aronco, S.; et al. Liposomal delivery of a Pin1 inhibitor complexed with cyclodextrins as new therapy for high-grade serous ovarian cancer. J. Control. Release 2018, 281, 1–10. [Google Scholar] [CrossRef]
- Luo, F.; Zeng, D.; Chen, R.; Zafar, A.; Weng, L.; Wang, W.; Tian, Y.; Hasan, M.; Shu, X. PEGylated dihydromyricetin-loaded nanoliposomes coated with tea saponin inhibit bacterial oxidative respiration and energy metabolism. Food Funct. 2021, 12, 9007–9017. [Google Scholar] [CrossRef]
- Bhatt, P.; Lalani, R.; Vhora, I.; Patil, S.; Amrutiya, J.; Misra, A.; Mashru, R. Liposomes encapsulating native and cyclodextrin enclosed paclitaxel: Enhanced loading efficiency and its pharmacokinetic evaluation. Int. J. Pharm. 2018, 536, 95–107. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Cirri, M.; Nerli, G.; Di Cesare Mannelli, L.; Ghelardini, C.; Mennini, N. Improvement of butamben anesthetic efficacy by the development of deformable liposomes bearing the drug as cyclodextrin complex. Pharmaceutics 2021, 13, 872. [Google Scholar] [CrossRef]
- Hasan, M.; Zafar, A.; Yousaf, M.; Gulzar, H.; Mehmood, K.; Hassan, S.G.; Saeed, A.; Yousaf, A.; Mazher, A.; Rongji, D.; et al. Synthesis of Loureirin B-Loaded Nanoliposomes for Pharmacokinetics in Rat Plasma. ACS Omega 2019, 4, 6914–6922. [Google Scholar] [CrossRef]
- Souri, J.; Almasi, H.; Hamishehkar, H.; Amjadi, S. Sodium caseinate-coated and β-cyclodextrin/vitamin E inclusion complex-loaded nanoliposomes: A novel stabilized nanocarrier. LWT 2021, 151, 112174. [Google Scholar] [CrossRef]
- Real, D.A.; Hoffmann, S.; Leonardi, D.; Goycoolea, F.M.; Salomon, C.J. A quality by design approach for optimization of Lecithin/Span® 80 based nanoemulsions loaded with hydrophobic drugs. J. Mol. Liq. 2021, 321, 114743. [Google Scholar] [CrossRef]
- Hou, K.; Xu, Y.; Cen, K.; Gao, C.; Feng, X.; Tang, X. Nanoemulsion of cinnamon essential oil Co-emulsified with hydroxypropyl-β-cyclodextrin and Tween-80: Antibacterial activity, stability and slow release performance. Food Biosci. 2021, 43, 101232. [Google Scholar] [CrossRef]
- Pangeni, R.; Choi, J.U.; Panth, V.K.; Byun, Y.; Park, J.W. Enhanced oral absorption of pemetrexed by ion-pairing complex formation with deoxycholic acid derivative and multiple nanoemulsion formulations: Preparation, characterization, and in vivo oral bioavailability and anticancer effect. Int. J. Nanomed. 2018, 13, 3329–3351. [Google Scholar] [CrossRef] [Green Version]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Singh, S.; Kushwaha, A.K.; Vuddanda, P.R.; Karunanidhi, P.; Singh, S.K. Development and evaluation of solid lipid nanoparticles of raloxifene hydrochloride for enhanced bioavailability. Biomed Res. Int. 2013, 2013, 584549. [Google Scholar] [CrossRef] [Green Version]
- Anderluzzi, G.; Lou, G.; Su, Y.; Perrie, Y. Scalable Manufacturing Processes for Solid Lipid Nanoparticles. Pharm. Nanotechnol. 2019, 7, 444–459. [Google Scholar] [CrossRef]
- Amasya, G.; Bakar-Ates, F.; Wintgens, V.; Amiel, C. Layer by layer assembly of core-corona structured solid lipid nanoparticles with β-cyclodextrin polymers. Int. J. Pharm. 2021, 592, 119994. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.; Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int. J. Pharm. 2010, 393, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Mennini, N.; Maestrelli, F.; Mura, P.; Ghelardini, C.; di Cesare Mannelli, L. Development and in vivo evaluation of an innovative “Hydrochlorothiazide-in Cyclodextrins-in Solid Lipid Nanoparticles” formulation with sustained release and enhanced oral bioavailability for potential hypertension treatment in pediatrics. Int. J. Pharm. 2017, 521, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Sarmento, B.; Ferreira, D.C.; Souto, E.B. Lipid-based colloidal carriers for peptide and protein delivery—Liposomes versus lipid nanoparticles. Int. J. Nanomed. 2007, 2, 595–607. [Google Scholar]
- Zhang, K.; Lv, S.; Li, X.; Feng, Y.; Li, X.; Liu, L.; Li, S.; Li, Y. Preparation, characterization, and in vivo pharmacokinetics of nanostructured lipid carriers loaded with oleanolic acid and gentiopicrin. Int. J. Nanomed. 2013, 8, 3227–3239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Improvement of curcuminoid bioaccessibility from turmeric by a nanostructured lipid carrier system. Food Chem. 2018, 251, 51–57. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Mannelli, L.D.C. Combined approach of cyclodextrin complexationand nanostructured lipid carriers for the development of a pediatric liquid oral dosage form of hydrochlorothiazide. Pharmaceutics 2018, 10, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, F.Q.; da Silva, J.K.R.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Lipid nanoparticles as carriers of cyclodextrin inclusion complexes: A promising approach for cutaneous delivery of a volatile essential oil. Colloids Surf. B Biointerfaces 2019, 182, 110382. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Polyakov, N.E.; Xu, W.; Su, W. Preparation of astaxanthin micelles self-assembled by a mechanochemical method from hydroxypropyl β-cyclodextrin and glyceryl monostearate with enhanced antioxidant activity. Int. J. Pharm. 2021, 605, 120799. [Google Scholar] [CrossRef]
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical Applications of Cellulose Ethers and Cellulose Ether Esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef]
- Priotti, J.; Codina, A.V.; Leonardi, D.; Vasconi, M.D.; Hinrichsen, L.I.; Lamas, M.C. Albendazole Microcrystal Formulations Based on Chitosan and Cellulose Derivatives: Physicochemical Characterization and In Vitro Parasiticidal Activity in Trichinella spiralis Adult Worms. AAPS PharmSciTech 2017, 18, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Ndong Ntoutoume, G.M.A.; Granet, R.; Mbakidi, J.P.; Brégier, F.; Léger, D.Y.; Fidanzi-Dugas, C.; Lequart, V.; Joly, N.; Liagre, B.; Chaleix, V.; et al. Development of curcumin–cyclodextrin/cellulose nanocrystals complexes: New anticancer drug delivery systems. Bioorg. Med. Chem. Lett. 2016, 26, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, M.; Msheik, Z.; Ndong-Ntoutoume, G.M.A.; Vignaud, L.; Richard, L.; Favreau, F.; Faye, P.A.; Sturtz, F.; Granet, R.; Vallat, J.M.; et al. Curcumin–cyclodextrin/cellulose nanocrystals improve the phenotype of Charcot-Marie-Tooth-1A transgenic rats through the reduction of oxidative stress. Free Radic. Biol. Med. 2020, 161, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Real, D.A.; Martinez, M.V.; Frattini, A.; Soazo, M.; Luque, A.G.; Biasoli, M.S.; Salomon, C.J.; Olivieri, A.C.; Leonardi, D. Design, characterization, and in vitro evaluation of antifungal polymeric films. AAPS PharmSciTech 2013, 14, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Yang, H.; Tang, B.; Wu, D.; Du, Q.; Xu, K.; Li, H. Dimethyl-β-cyclodextrin/salazosulfapyridine inclusion complex-loaded chitosan nanoparticles for sustained release. Carbohydr. Polym. 2017, 156, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Yin, Y.; Shang, L.; Wu, T.; Zhang, D.; Kong, M.; Zhao, Y.; He, Y.; Tan, S.; Guo, Y.; et al. Tumor Microenvironment Responsive Nanogel for the Combinatorial Antitumor Effect of Chemotherapy and Immunotherapy. Nano Lett. 2017, 17, 6366–6375. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, H.; Choi, E.S.; Park, M.H.; Ryu, J.H. Hyaluronic acid-coated nanomedicine for targeted cancer therapy. Pharmaceutics 2019, 11, 301. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, Y.M.; Chen, Y.; Chen, J.T.; Liu, Y. Polysaccharide-based Noncovalent Assembly for Targeted Delivery of Taxol. Sci. Rep. 2016, 6, 19212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Wang, M.; Huang, L.; Wu, Z.; Wang, W.; Zafar, A.; Tian, Y.; Hasan, M.; Shu, X. Synthesis of Zinc Oxide Eudragit FS30D Nanohybrids: Structure, Characterization, and Their Application as an Intestinal Drug Delivery System. ACS Omega 2020, 5, 11799–11808. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.S.B. Selection and optimization of nano-formulation of P-glycoprotein inhibitor for reversal of doxorubicin resistance in COLO205 cells. J. Pharm. Pharmacol. 2017, 69, 834–843. [Google Scholar] [CrossRef]
- Maged, A.; Mahmoud, A.A.; Ghorab, M.M. Nano spray drying technique as a novel approach to formulate stable econazole nitrate nanosuspension formulations for ocular use. Mol. Pharm. 2016, 13, 2951–2965. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Guo, X.; Shi, Y.; Li, W.; Zhang, G. Novel amino-β-Cyclodextrins containing polymers: Fabrication, characterization, and biological evaluation. Colloids Surf. B Biointerfaces 2020, 196, 111311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, Z.H.; Li, L.; Luo, Y.L.; Xu, F.; Chen, Y. Dual Stimuli-Responsive Supramolecular Self-Assemblies Based on the Host-Guest Interaction between β-Cyclodextrin and Azobenzene for Cellular Drug Release. Mol. Pharm. 2020, 17, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Solanki, A.; Joshi, A.; Devkar, R.; Seshadri, S.; Thakore, S. β-cyclodextrin based dual-responsive multifunctional nanotheranostics for cancer cell targeting and dual drug delivery. Carbohydr. Polym. 2019, 206, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, M.; García-Zubiri, I.X.; Isasi, J.R. History of cyclodextrin-based polymers in food and pharmacy: A review. Environ. Chem. Lett. 2021, 19, 3465–3476. [Google Scholar] [CrossRef] [PubMed]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005, 6, E329–E357. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Mu, J.; Zeng, L.; Lin, J.; Nie, Z.; Jiang, X.; Huang, P. Stimuli-responsive cyclodextrin-based nanoplatforms for cancer treatment and theranostics. Mater. Horiz. 2019, 6, 846–870. [Google Scholar] [CrossRef]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin based nanoparticles for drug delivery and theranostics. Adv. Pharm. Bull. 2020, 10, 166–183. [Google Scholar] [CrossRef] [Green Version]
- Yakavets, I.; Guereschi, C.; Lamy, L.; Kravchenko, I.; Lassalle, H.P.; Zorin, V.; Bezdetnaya, L. Cyclodextrin nanosponge as a temoporfin nanocarrier: Balancing between accumulation and penetration in 3D tumor spheroids. Eur. J. Pharm. Biopharm. 2020, 154, 33–42. [Google Scholar] [CrossRef]
- Giglio, V.; Viale, M.; Bertone, V.; Maric, I.; Vaccarone, R.; Vecchio, G. Cyclodextrin polymers as nanocarriers for sorafenib. Investig. New Drugs 2018, 36, 370–379. [Google Scholar] [CrossRef]
- Viale, M.; Tosto, R.; Giglio, V.; Pappalardo, G.; Oliveri, V.; Maric, I.; Mariggiò, M.A.; Vecchio, G. Cyclodextrin polymers decorated with RGD peptide as delivery systems for targeted anti-cancer chemotherapy. Investig. New Drugs 2019, 37, 771–778. [Google Scholar] [CrossRef]
- Cordaro, A.; Zagami, R.; Malanga, M.; Venkatesan, J.K.; Alvarez-Lorenzo, C.; Cucchiarini, M.; Piperno, A.; Mazzaglia, A. Cyclodextrin cationic polymer-based nanoassemblies to manage inflammation by intra-articular delivery strategies. Nanomaterials 2020, 10, 1712. [Google Scholar] [CrossRef] [PubMed]
- Costa-Gouveia, J.; Pancani, E.; Jouny, S.; Machelart, A.; Delorme, V.; Salzano, G.; Iantomasi, R.; Piveteau, C.; Queval, C.J.; Song, O.R.; et al. Combination therapy for tuberculosis treatment: Pulmonary administration of ethionamide and booster co-loaded nanoparticles. Sci. Rep. 2017, 7, 5390. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Wankar, J.; Ottani, S.; Villemagne, B.; Baulard, A.R.; Willand, N.; Brodin, P.; Manet, I.; Gref, R. Cyclodextrin-based nanocarriers containing a synergic drug combination: A potential formulation for pulmonary administration of antitubercular drugs. Int. J. Pharm. 2017, 531, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Thatiparti, T.R.; Juric, D.; Von Recum, H.A. Pseudopolyrotaxane Formation in the Synthesis of Cyclodextrin Polymers: Effects on Drug Delivery, Mechanics, and Cell Compatibility. Bioconjug. Chem. 2017, 28, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Moin, A.; Roohi, N.K.F.; Rizvi, S.M.D.; Ashraf, S.A.; Siddiqui, A.J.; Patel, M.; Ahmed, S.M.; Gowda, D.V.; Adnan, M. Design and formulation of polymeric nanosponge tablets with enhanced solubility for combination therapy. RSC Adv. 2020, 10, 34869–34884. [Google Scholar] [CrossRef]
- Shringirishi, M.; Mahor, A.; Gupta, R.; Prajapati, S.K.; Bansal, K.; Kesharwani, P. Fabrication and characterization of nifedipine loaded β-cyclodextrin nanosponges: An in vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2017, 41, 344–350. [Google Scholar] [CrossRef]
- Zidan, M.F.; Ibrahim, H.M.; Afouna, M.I.; Ibrahim, E.A. In vitro and in vivo evaluation of cyclodextrin-based nanosponges for enhancing oral bioavailability of atorvastatin calcium. Drug Dev. Ind. Pharm. 2018, 44, 1243–1253. [Google Scholar] [CrossRef]
- Amin, O.M.; Ammar, A.; Eladawy, S.A. Febuxostat loaded β-cyclodextrin based nanosponge tablet: An in vitro and in vivo evaluation. J. Pharm. Investig. 2020, 50, 399–411. [Google Scholar] [CrossRef]
- Salazar, S.; Yutronic, N.; Kogan, M.J.; Jara, P. Cyclodextrin nanosponges inclusion compounds associated with gold nanoparticles for potential application in the photothermal release of melphalan and cytoxan. Int. J. Mol. Sci. 2021, 22, 6446. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Riveros, A.; Yutronic, N.; Lang, E.; Chornik, B.; Guerrero, S.; Samitier, J.; Jara, P.; Kogan, M. Photothermally Controlled Methotrexate Release System Using β-Cyclodextrin and Gold Nanoparticles. Nanomaterials 2018, 8, 985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asela, I.; Donoso-González, O.; Yutronic, N.; Sierpe, R. β-Cyclodextrin-Based Nanosponges Functionalized with Drugs and Gold Nanoparticles. Pharmaceutics 2021, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Chan, H.F.; Shi, B.; Li, M.; Leong, K.W. Light: A Magical Tool for Controlled Drug Delivery. Adv. Funct. Mater. 2020, 30, 2005029. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Advances in drug delivery nanosystems using graphene-based materials and carbon nanotubes. Materials 2021, 14, 1059. [Google Scholar] [CrossRef]
- Hoseini-Ghahfarokhi, M.; Mirkiani, S.; Mozaffari, N.; Abdolahi Sadatlu, M.A.; Ghasemi, A.; Abbaspour, S.; Akbarian, M.; Farjadian, F.; Karimi, M. Applications of graphene and graphene oxide in smart drug/gene delivery: Is the world still flat? Int. J. Nanomed. 2020, 15, 9469–9496. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.; Yu, L.; Huang, Y. Light-triggered C60 release from a graphene/cyclodextrin nanoplatform for the protection of cytotoxicity induced by nitric oxide. J. Mater. Chem. B 2018, 6, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, M.Y.; Wang, L.L.; Yuan, H.; Wang, M.; Wu, Y.; Li, T. Synthesis of novel cyclodextrin-modified reduced graphene oxide composites by a simple hydrothermal method. RSC Adv. 2018, 8, 37623–37630. [Google Scholar] [CrossRef] [Green Version]
- Das, M.; Nariya, P.; Joshi, A.; Vohra, A.; Devkar, R.; Seshadri, S.; Thakore, S. Carbon nanotube embedded cyclodextrin polymer derived injectable nanocarrier: A multiple faceted platform for stimulation of multi-drug resistance reversal. Carbohydr. Polym. 2020, 247, 116751. [Google Scholar] [CrossRef]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Lim, K.T. Graphene oxide-based stimuli-responsive platforms for biomedical applications. Molecules 2021, 26, 2797. [Google Scholar] [CrossRef] [PubMed]
- Borandeh, S.; Abdolmaleki, A.; Abolmaali, S.S.; Tamaddon, A.M. Synthesis, structural and in-vitro characterization of β-cyclodextrin grafted L-phenylalanine functionalized graphene oxide nanocomposite: A versatile nanocarrier for pH-sensitive doxorubicin delivery. Carbohydr. Polym. 2018, 201, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Pooresmaeil, M.; Namazi, H. β-Cyclodextrin grafted magnetic graphene oxide applicable as cancer drug delivery agent: Synthesis and characterization. Mater. Chem. Phys. 2018, 218, 62–69. [Google Scholar] [CrossRef]
- Siriviriyanun, A.; Tsai, Y.J.; Voon, S.H.; Kiew, S.F.; Imae, T.; Kiew, L.V.; Looi, C.Y.; Wong, W.F.; Lee, H.B.; Chung, L.Y. Cyclodextrin- and dendrimer-conjugated graphene oxide as a nanocarrier for the delivery of selected chemotherapeutic and photosensitizing agents. Mater. Sci. Eng. C 2018, 89, 307–315. [Google Scholar] [CrossRef]
- Wang, P.; Huang, C.; Xing, Y.; Fang, W.; Ren, J.; Yu, H.; Wang, G. NIR-Light- and pH-Responsive Graphene Oxide Hybrid Cyclodextrin-Based Supramolecular Hydrogels. Langmuir 2019, 35, 1021–1031. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Q.; Liu, Y. Alternating Magnetic Field Controlled Targeted Drug Delivery Based on Graphene Oxide-Grafted Nanosupramolecules. Chem.—A Eur. J. 2020, 26, 13698–13703. [Google Scholar] [CrossRef]
- Sattari, S.; Tehrani, A.D.; Adeli, M.; Soleimani, K.; Rashidipour, M. Fabrication of new generation of co-delivery systems based on graphene-g-cyclodextrin/chitosan nanofiber. Int. J. Biol. Macromol. 2020, 156, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Mikhraliieva, A.; Zaitsev, V.; Tkachenko, O.; Nazarkovsky, M.; Xing, Y.; Benvenutti, E.V. Graphene oxide quantum dots immobilized on mesoporous silica: Preparation, characterization and electroanalytical application. RSC Adv. 2020, 10, 31305–31315. [Google Scholar] [CrossRef]
- Yang, T.; Huang, J.L.; Wang, Y.T.; Zheng, A.Q.; Shu, Y.; Wang, J.H. β-Cyclodextrin-Decorated Carbon Dots Serve as Nanocarriers for Targeted Drug Delivery and Controlled Release. ChemNanoMat 2019, 5, 479–487. [Google Scholar] [CrossRef]
- Agbo, C.P.; Ugwuanyi, T.C.; Ugwuoke, W.I.; McConville, C.; Attama, A.A.; Ofokansi, K.C. Intranasal artesunate-loaded nanostructured lipid carriers: A convenient alternative to parenteral formulations for the treatment of severe and cerebral malaria. J. Control. Release 2021, 334, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Zheng, J.; Lv, P.; Zhang, D.; Zheng, X.; Zhang, Y.; Liao, R. Controlled Drug Release from Cyclodextrin-Gated Mesoporous Silica Nanoparticles Based on Switchable Host-Guest Interactions. Bioconjugate Chem. 2018, 29, 2884–2891. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Xue, Z.; Sun, P.; Shen, K.; Wang, S.; Jia, J. Visible-light-triggered supramolecular valves based on β-cyclodextrin-modified mesoporous silica nanoparticles for controlled drug release. RSC Adv. 2019, 9, 17179–17182. [Google Scholar] [CrossRef] [Green Version]
- Velasco-Aguirre, C.; Morales, F.; Gallardo-Toledo, E.; Guerrero, S.; Giralt, E.; Araya, E.; Kogan, M.J. Peptides and proteins used to enhance gold nanoparticle delivery to the brain: Preclinical approaches. Int. J. Nanomed. 2015, 10, 4919–4936. [Google Scholar]
- Kogan, M.J.; Bastus, N.G.; Amigo, R.; Grillo-Bosch, D.; Araya, E.; Turiel, A.; Labarta, A.; Giralt, E.; Puntes, V.F. Nanoparticle-mediated local and remote manipulation of protein aggregation. Nano Lett. 2006, 6, 110–115. [Google Scholar] [CrossRef]
- Lara, P.; Palma-Florez, S.; Salas-Huenuleo, E.; Polakovicova, I.; Guerrero, S.; Lobos-Gonzalez, L.; Campos, A.; Muñoz, L.; Jorquera-Cordero, C.; Varas-Godoy, M.; et al. Gold nanoparticle based double-labeling of melanoma extracellular vesicles to determine the specificity of uptake by cells and preferential accumulation in small metastatic lung tumors. J. Nanobiotechnol. 2020, 18, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jara-Guajardo, P.; Cabrera, P.; Celis, F.; Soler, M.; Berlanga, I.; Parra-Muñoz, N.; Acosta, G.; Albericio, F.; Guzman, F.; Campos, M.; et al. Gold nanoparticles mediate improved detection of β-amyloid aggregates by fluorescence. Nanomaterials 2020, 10, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prades, R.; Guerrero, S.; Araya, E.; Molina, C.; Salas, E.; Zurita, E.; Selva, J.; Egea, G.; López-Iglesias, C.; Teixidó, M.; et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials 2012, 33, 7194–7205. [Google Scholar] [CrossRef] [PubMed]
- Ruff, J.; Hüwel, S.; Kogan, M.J.; Simon, U.; Galla, H.J. The effects of gold nanoparticles functionalized with ß-amyloid specific peptides on an in vitro model of blood–brain barrier. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Li, Z.; Fu, F.; Chen, X.; Song, J.; Yang, H. Stimuli-Responsive Plasmonic Assemblies and Their Biomedical Applications. Nano Today 2021, 36, 101014. [Google Scholar] [CrossRef]
- Morales-Zavala, F.; Arriagada, H.; Hassan, N.; Velasco, C.; Riveros, A.; Álvarez, A.R.; Minniti, A.N.; Rojas-Silva, X.; Muñoz, L.L.; Vasquez, R.; et al. Peptide multifunctionalized gold nanorods decrease toxicity of β-amyloid peptide in a Caenorhabditis elegans model of Alzheimer’s disease. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Ruff, J.; Hassan, N.; Morales-Zavala, F.; Steitz, J.; Araya, E.; Kogan, M.J.; Simon, U. CLPFFD-PEG functionalized NIR-absorbing hollow gold nanospheres and gold nanorods inhibit β-amyloid aggregation. J. Mater. Chem. B 2018, 6, 2432–2443. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Cordero, M.L.; Sierpe, R.; Almada, M.; Juárez, J.; Valdez, M.; Riveros, A.; Vargas, E.; Abou-Hassan, A.; Ruso, J.M.; et al. Peptide functionalized magneto-plasmonic nanoparticles obtained by microfluidics for inhibition of β-amyloid aggregation. J. Mater. Chem. B 2018, 6, 5091–5099. [Google Scholar] [CrossRef]
- Tapia-Arellano, A.; Gallardo-Toledo, E.; Ortiz, C.; Henríquez, J.; Feijóo, C.G.; Araya, E.; Sierpe, R.; Kogan, M.J. Functionalization with PEG/Angiopep-2 peptide to improve the delivery of gold nanoprisms to central nervous system: In vitro and in vivo studies. Mater. Sci. Eng. C 2021, 121, 111785. [Google Scholar] [CrossRef]
- Morales-Zavala, F.; Jara-Guajardo, P.; Chamorro, D.; Riveros, A.L.; Chandia-Cristi, A.; Salgado, N.; Pismante, P.; Giralt, E.; Sánchez-Navarro, M.; Araya, E.; et al. In vivo micro computed tomography detection and decrease in amyloid load by using multifunctionalized gold nanorods: A neurotheranostic platform for Alzheimer’s disease. Biomater. Sci. 2021, 9, 4178–4190. [Google Scholar] [CrossRef]
- Adura, C.; Guerrero, S.; Salas, E.; Medel, L.; Riveros, A.; Mena, J.; Arbiol, J.; Albericio, F.; Giralt, E.; Kogan, M.J. Stable conjugates of peptides with gold nanorods for biomedical applications with reduced effects on cell viability. ACS Appl. Mater. Interfaces 2013, 5, 4076–4085. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Graña, S.; Pérez-Juste, J.; Hervés, P. Cyclodextrins and inorganic nanoparticles: Another tale of synergy. Adv. Colloid Interface Sci. 2021, 288, 102338. [Google Scholar] [CrossRef]
- Donoso-González, O.; Lodeiro, L.; Aliaga, Á.E.; Laguna-Bercero, M.A.; Bollo, S.; Kogan, M.J.; Yutronic, N.; Sierpe, R. Functionalization of Gold Nanostars with Cationic β-Cyclodextrin-Based Polymer for Drug Co-Loading and SERS Monitoring. Pharmaceutics 2021, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.I.; Uyar, T. One-step green synthesis of antibacterial silver nanoparticles embedded in electrospun cyclodextrin nanofibers. Carbohydr. Polym. 2019, 207, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Ramasamy, S.; Enoch, I.V.M.V.; Rex Jeya Rajkumar, S. Polymeric cyclodextrin-dextran spooled nickel ferrite nanoparticles: Expanded anticancer efficacy of loaded camptothecin. Mater. Lett. 2020, 261, 127114. [Google Scholar] [CrossRef]
- Maxwell, T.; Nogueira Campos, M.G.; Smith, S.; Doomra, M.; Thwin, Z.; Santra, S. Quantum dots. In Nanoparticles for Biomedical Applications: Fundamental Concepts, Biological Interactions and Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–265. ISBN 9780128166628. [Google Scholar]
- Shu, C.; Li, R.; Guo, J.; Ding, L.; Zhong, W. New generation of β-cyclodextrin-chitosan nanoparticles encapsulated quantum dots loaded with anticancer drug for tumor-target drug delivery and imaging of cancer cells. J. Nanopart. Res. 2013, 15, 1927. [Google Scholar] [CrossRef]
- Li, Z.; Song, N.; Yang, Y.W. Stimuli-Responsive Drug-Delivery Systems Based on Supramolecular Nanovalves. Matter 2019, 1, 345–368. [Google Scholar] [CrossRef] [Green Version]
- Han, R.L.; Shi, J.H.; Liu, Z.J.; Hou, Y.F.; Wang, Y. Near-Infrared Light-Triggered Hydrophobic-to-Hydrophilic Switch Nanovalve for On-Demand Cancer Therapy. ACS Biomater. Sci. Eng. 2018, 4, 3478–3486. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Stoddart, J.F. Cyclodextrin Metal-Organic Frameworks and Their Applications. Acc. Chem. Res. 2021, 54, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Hartlieb, K.J.; Ferris, D.P.; Holcroft, J.M.; Kandela, I.; Stern, C.L.; Nassar, M.S.; Botros, Y.Y.; Stoddart, J.F. Encapsulation of Ibuprofen in CD-MOF and Related Bioavailability Studies. Mol. Pharm. 2017, 14, 1831–1839. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; McGuirk, C.M.; D’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal–Organic Framework Nanoparticles. Adv. Mater. 2018, 30, 1800202. [Google Scholar] [CrossRef]
- Agostoni, V.; Horcajada, P.; Noiray, M.; Malanga, M.; Aykaç, A.; Jicsinszky, L.; Vargas-Berenguel, A.; Semiramoth, N.; Daoud-Mahammed, S.; Nicolas, V.; et al. A “green” strategy to construct non-covalent, stable and bioactive coatings on porous MOF nanoparticles. Sci. Rep. 2015, 5, 7925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoee, S.; Nouri, A. Preparation of Janus nanoparticles and its application in drug delivery. In Design and Development of New Nanocarriers; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 145–180. [Google Scholar]
- Hernández Montoto, A.; Llopis-Lorente, A.; Gorbe, M.; Terrés, J.M.; Cao-Milán, R.; Díaz de Greñu, B.; Alfonso, M.; Ibañez, J.; Marcos, M.D.; Orzáez, M.; et al. Janus Gold Nanostars–Mesoporous Silica Nanoparticles for NIR-Light-Triggered Drug Delivery. Chem.—A Eur. J. 2019, 25, 8471–8478. [Google Scholar] [CrossRef]
- Sagitha, P.; Reshmi, C.R.; Sundaran, S.P.; Binoy, A.; Mishra, N.; Sujith, A. β-Cyclodextrin functionalized polyurethane nano fibrous membranes for drug delivery. J. Drug Deliv. Sci. Technol. 2021, 65, 102759. [Google Scholar] [CrossRef]
- Stjern, L.; Voittonen, S.; Weldemichel, R.; Thuresson, S.; Agnes, M.; Benkovics, G.; Fenyvesi, É.; Malanga, M.; Yannakopoulou, K.; Feiler, A.; et al. Cyclodextrin-mesoporous silica particle composites for controlled antibiotic release. A proof of concept toward colon targeting. Int. J. Pharm. 2017, 531, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Velasco Delgadillo, R.M.; Barrera, E.V. Covalently functionalized carbon nano-onions integrated gelatin methacryloyl nanocomposite hydrogel containing γ-cyclodextrin as drug carrier for high-performance ph-triggered drug release. Pharmaceuticals 2021, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Rao, V.M.; Zannou, E.A.; Zia, V. Mechanisms of drug release from cyclodextrin complexes. Adv. Drug Deliv. Rev. 1999, 36, 3–16. [Google Scholar] [CrossRef]
- Okubo, M.; Iohara, D.; Anraku, M.; Higashi, T.; Uekama, K.; Hirayama, F. A thermoresponsive hydrophobically modified hydroxypropylmethylcellulose/cyclodextrin injectable hydrogel for the sustained release of drugs. Int. J. Pharm. 2020, 575, 118845. [Google Scholar] [CrossRef] [PubMed]
- Repasky, E.A.; Evans, S.S.; Dewhirst, M.W. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol. Res. 2013, 1, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Li, G.; Zhou, H.; Ma, S.; Guo, L.; Liu, X. Temperature and H2O2-operated nano-valves on mesoporous silica nanoparticles for controlled drug release and kinetics. Colloids Surf. B Biointerfaces 2020, 187, 110643. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Tian, X.; Deng, H.; Wang, Y.; Jiang, X. Dialdehyde-β-cyclodextrin-crosslinked carboxymethyl chitosan hydrogel for drug release. Carbohydr. Polym. 2020, 231, 115678. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, I.; Akhtar, M.; Ahmad, R.; Sadaquat, H.; Noreen, S.; Batool, A.; Khan, S.U. Novel multiparticulate pH triggered delayed release chronotherapeutic drug delivery of celecoxib-β-cyclodextrin inclusion complexes by using Box-Behnken design. Eur. J. Pharm. Sci. 2020, 146, 105254. [Google Scholar] [CrossRef]
- Wang, R.; Tian, Y.; Wang, J.; Song, W.; Cong, Y.; Wei, X.; Mei, Y.; Miyatake, H.; Ito, Y.; Chen, Y.M. Biomimetic Glucose Trigger-Insulin Release System Based on Hydrogel Loading Bidentate β-Cyclodextrin. Adv. Funct. Mater. 2021, 31, 2104488. [Google Scholar] [CrossRef]
- Xu, X.; Shang, H.; Zhang, T.; Shu, P.; Liu, Y.; Xie, J.; Zhang, D.; Tan, H.; Li, J. A stimuli-responsive insulin delivery system based on reversible phenylboronate modified cyclodextrin with glucose triggered host-guest interaction. Int. J. Pharm. 2018, 548, 649–658. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Z.; Sun, W.; Yang, Y.; Jin, H.; Qiu, L.; Chen, J.; Chen, J. Co-responsive smart cyclodextrin-gated mesoporous silica nanoparticles with ligand-receptor engagement for anti-cancer treatment. Mater. Sci. Eng. C 2019, 103, 109831. [Google Scholar] [CrossRef] [PubMed]
- Enoch, I.V.M.V.; Ramasamy, S.; Mohiyuddin, S.; Gopinath, P.; Manoharan, R. Cyclodextrin-PEG conjugate-wrapped magnetic ferrite nanoparticles for enhanced drug loading and release. Appl. Nanosci. 2018, 8, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Liu, X.; Liu, H.; Dong, D.; Li, L.; Jiang, L.; Huang, M.; Ding, C. Novel pH-Triggered Doxorubicin-Releasing Nanoparticles Self-Assembled by Functionalized Î−Cyclodextrin and Amphiphilic Phthalocyanine for Anticancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 10674–10688. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.R.; Sabatini, D.M. Cancer cell metabolism: One hallmark, many faces. Cancer Discov. 2012, 2, 881–898. [Google Scholar] [CrossRef] [Green Version]
- Zamarin, D.; Holmgaard, R.B.; Subudhi, S.K.; Park, J.S.; Mansour, M.; Palese, P.; Merghoub, T.; Wolchok, J.D.; Allison, J.P. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014, 6, 226ra32. [Google Scholar] [CrossRef] [Green Version]
- Bedard, P.L.; Hansen, A.R.; Ratain, M.J.; Siu, L.L. Tumour heterogeneity in the clinic. Nature 2013, 501, 355–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbora, A.; Bohar, O.; Sivan, A.A.; Magory, E.; Nause, A.; Minnes, R. Higher pulse frequency of near-infrared laser irradiation increases penetration depth for novel biomedical applications. PLoS ONE 2021, 16, e0245350. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, J.; Steenekamp, J.; Steyn, D.; Hamman, J. The role of functional excipients in solid oral dosage forms to overcome poor drug dissolution and bioavailability. Pharmaceutics 2020, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Tuomela, A.; Hirvonen, J.; Peltonen, L. Stabilizing agents for drug nanocrystals: Effect on bioavailability. Pharmaceutics 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.L.; Dinu-Pîrvu, C.E. Strategies for improving ocular drug bioavailability and cornealwound healing with chitosan-based delivery systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukumar, U.K.; Bose, R.J.C.; Malhotra, M.; Babikir, H.A.; Afjei, R.; Robinson, E.; Zeng, Y.; Chang, E.; Habte, F.; Sinclair, R.; et al. Intranasal delivery of targeted polyfunctional gold–iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials 2019, 218, 119342. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liang, Y.; Liu, L.; Yin, M.; Wang, A.; Sun, K.; Li, Y.; Shi, Y. Research on the fate of polymeric nanoparticles in the process of the intestinal absorption based on model nanoparticles with various characteristics: Size, surface charge and pro-hydrophobics. J. Nanobiotechnol. 2021, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Flynn, E. Drug bioavailability. In xPharm: The Comprehensive Pharmacology Reference; StatPearls Publishing: Treasure Island, FL, USA, 2007; pp. 1–2. ISBN 9780080552323. [Google Scholar]
- Mudie, D.M.; Stewart, A.M.; Rosales, J.A.; Adam, M.S.; Morgen, M.M.; Vodak, D.T. In vitro-in silico tools for streamlined development of acalabrutinib amorphous solid dispersion tablets. Pharmaceutics 2021, 13, 1257. [Google Scholar] [CrossRef]
- Lombardo, F.; Bentzien, J.; Berellini, G.; Muegge, I. In Silico Models of Human PK Parameters. Prediction of Volume of Distribution Using an Extensive Data Set and a Reduced Number of Parameters. J. Pharm. Sci. 2021, 110, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Murad, N.; Pasikanti, K.K.; Madej, B.D.; Minnich, A.; McComas, J.M.; Crouch, S.; Polli, J.W.; Weber, A.D. Predicting volume of distribution in humans: Performance of in silico methods for a large set of structurally diverse clinical compounds. Drug Metab. Dispos. 2021, 49, 169–178. [Google Scholar] [CrossRef]
- Arafat, M.; Sarfraz, M.; Aburuz, S. Development and in vitro evaluation of controlled release viagra® containing poloxamer-188 using gastroplusTM pbpk modeling software for in vivo predictions and pharmacokinetic assessments. Pharmaceuticals 2021, 14, 479. [Google Scholar] [CrossRef]
- Spini, A.; Donnini, S.; Pantziarka, P.; Crispino, S.; Ziche, M. Repurposing of drugs for triple negative breast cancer: An overview. Ecancermedicalscience 2020, 14, 1071. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Real, D.A.; Bolaños, K.; Priotti, J.; Yutronic, N.; Kogan, M.J.; Sierpe, R.; Donoso-González, O. Cyclodextrin-Modified Nanomaterials for Drug Delivery: Classification and Advances in Controlled Release and Bioavailability. Pharmaceutics 2021, 13, 2131. https://doi.org/10.3390/pharmaceutics13122131
Real DA, Bolaños K, Priotti J, Yutronic N, Kogan MJ, Sierpe R, Donoso-González O. Cyclodextrin-Modified Nanomaterials for Drug Delivery: Classification and Advances in Controlled Release and Bioavailability. Pharmaceutics. 2021; 13(12):2131. https://doi.org/10.3390/pharmaceutics13122131
Chicago/Turabian StyleReal, Daniel Andrés, Karen Bolaños, Josefina Priotti, Nicolás Yutronic, Marcelo J. Kogan, Rodrigo Sierpe, and Orlando Donoso-González. 2021. "Cyclodextrin-Modified Nanomaterials for Drug Delivery: Classification and Advances in Controlled Release and Bioavailability" Pharmaceutics 13, no. 12: 2131. https://doi.org/10.3390/pharmaceutics13122131
APA StyleReal, D. A., Bolaños, K., Priotti, J., Yutronic, N., Kogan, M. J., Sierpe, R., & Donoso-González, O. (2021). Cyclodextrin-Modified Nanomaterials for Drug Delivery: Classification and Advances in Controlled Release and Bioavailability. Pharmaceutics, 13(12), 2131. https://doi.org/10.3390/pharmaceutics13122131