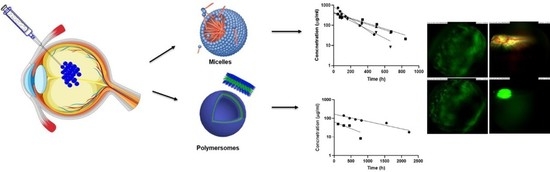
Intravitreal Polymeric Nanocarriers with Long Ocular Retention and Targeted Delivery to the Retina and Optic Nerve Head Region
Abstract
:1. Introduction
2. Materials
3. Methods
3.1. Synthesis and Characterization of Block Copolymers
3.2. Preparation and Physical Properties of Self-Assembling Nanoparticles
3.3. In Vivo Experiments in Rabbits
4. Results
4.1. Characterization of Polymeric Micelles and Polymersomes
4.2. Polymersome Interactions with Ex Vivo Vitreous Humour
4.3. Particle Kinetics In Vivo after Intravitreal Injection
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbr. | Full Names |
| ACN | acetonitrile |
| AF4 | asymmetric flow field flow fractionation |
| BODIPY | boron-dipyrromethene; |
| CMC | critical micellar concentration |
| Dv | diffusion coefficient in vitreous |
| DCM | dichloromethane |
| DLS | dynamic light scattering |
| DMEM | Dulbecco′s modified eagle′s medium |
| DPBS | Dulbecco’s phosphate buffered saline |
| ECGS | endothelial cell growth supplement |
| FBS | fetal bovine serum |
| GPC | gel permeation chromatography |
| MALS | multi-angle light scattering |
| MSD | mean square displacement |
| NMR | nuclear magnetic resonance |
| PEG | Poly(ethylene glycol) |
| PBS | phosphate buffered saline |
| PCL | poly(ε-caprolactone) |
| PTMC | poly(trimethylene carbonate) |
| ROP | ring-opening polymerization |
| RT | room temperature |
| τ | time delay for the calculated movement |
| tBOC | tert-butyloxycarbonyl |
| TFA | trifluoroacetic acid |
| THF | tetrahydrofuran |
| TMC | trimethylene carbonate |
References
- Yerxa, B. Progress in Inherited Retinal Disease Drug Discovery and Development: A Foundation’s Perspective. Pharm. Res. 2018, 35, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Amo, E.M.; Rimpelä, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Prog. Ret. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Mishima, S. Clinical pharmacokinetics of the eye. Investig. Ophthalmol. Vis. Sci. 1981, 21, 504–541. [Google Scholar]
- Intravitreal Injections—Frequently Asked Questions. Available online: https://retinavitreous.com/treatments/intravitreal_injections.php (accessed on 25 March 2021).
- Del Amo, E.M.; Urtti, A. Current and Future Ophthalmic Drug Delivery Systems. A Shift to the Posterior Segment. Drug Discov. Today 2008, 13, 135–143. [Google Scholar] [CrossRef]
- Wagner, V.; Dullaart, A.; Bock, A.K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotech. 2006, 24, 1211–1217. [Google Scholar] [CrossRef]
- Discher, M.; Won, Y.; Ege, D.S.; Lee, J.C.M.; Bates, F.S.; Disher, D.E.; Hammer, D.A. Polymersomes: Tough Vesicles Made from Diblock Copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Feijen, J. Polymersomes for drug delivery: Design, formation and characterization. J. Control. Rel. 2012, 161, 473–483. [Google Scholar] [CrossRef]
- Zhou, W. Biodegradable polymersomes for controlled drug release. J. Control. Rel. 2008, 132, e35. [Google Scholar] [CrossRef]
- Van Dongen, S.F.M.; de Hoog, H.P.M.; Peters, R.; Nallani, M.; Nolte, R.J.M.; van Hest, J.C. Biohybrid polymer capsules. Chem. Rev. 2009, 109, 6212–6274. [Google Scholar] [CrossRef] [PubMed]
- Kompella, U.B.; Amrite, A.C.; Ravi, R.P.; Durazo, S.A. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog. Ret. Eye Res. 2013, 36, 172–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, F.; Nan, K.; Lee, S.; Beadle, J.R.; Hou, H.; Freeman, W.R.; Hostetler, K.Y.; Cheng, L. Micelle formulation of Hexadecyloxypropyl-Cidofovir (HDP-CDV) as an intravitreal long-lasting delivery system. Eur. J. Pharm. Biopharm. 2015, 89, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Rel. 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; He, Z.; Zhang, Z.; Yu, X.; Song, Z.; Li, X. Intravitreal injection of rapamycin-loaded polymeric micelles for inhibition of ocular inflammation in rat model. Int. J. Pharm. 2016, 513, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Ridolfo, R.; Ede, B.C.; Diamanti, P.; White, P.B.; Perriman, A.W.; van Hest, J.C.M.; Blair, A.; Williams, D.S. Biodegradable, Drug-Loaded Nanovectors via Direct Hydration as a New Platform for Cancer Therapeutics. Small 2018, 14, 1703774. [Google Scholar] [CrossRef] [PubMed]
- Van Oppen, L.; Abdelmohsen, L.K.E.A.; van Emst-de Vries, S.E.; Welzen, P.L.W.; Wilson, D.A.; Smeitink, J.A.M.; Koopman, W.J.H.; Brock, R.; Willems, P.H.G.M.; Williams, D.S.; et al. Biodegradable Synthetic Organelles Demonstrate ROS Shielding in Human-Complex-I-Deficient Fibroblasts. ACS Cent. Sci. 2018, 4, 917–928. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Sarkhel, S.; Peltoniemi, J.; Broadbridge, R.; Tuomainen, M.; Auriola, S.; Urtti, A. Differentially cleaving peptides as a strategy for controlled drug release in human retinal pigment epithelial cells. J. Control. Rel. 2017, 251, 37–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimpelä, A.K.; Reunanen, S.; Hagström, M.; Kidron, H.; Urtti, A. Binding of Small Molecule Drugs to Porcine Vitreous Humor. Mol. Pharmaceut. 2018, 15, 2174–2179. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, S.; Kari, O.K.; Turunen, T.; Lajunen, T.; Schmitt, M.; Lehtinen, J.; Tasaka, F.; Parkkila, P.; Ndika, J.; Viitala, T.; et al. Diffusion and protein corona formation of lipid-based nanoparticles in vitreous humor: Profiling and pharmacokinetic considerations. Mol. Pharmaceut. 2021, 18, 699–713. [Google Scholar] [CrossRef]
- Ridolfo, R.; Tavakoli, S.; Junnuthula, V.; Williams, D.; Urtti, A.; van Hest, J. Exploring the impact of morphology on the properties of biodegradable nanoparticles and their diffusion in complex biological medium. Biomacromolecules 2021, 22, 126–133. [Google Scholar] [CrossRef]
- Barza, M.; Stuart, M.; Szoka, F. Effect of size and lipid composition on the pharmacokinetics of intravitreal liposomes. Investig. Ophthalmol. Vis. Sci. 1987, 28, 893–900. [Google Scholar]
- Fischella, R.; Peyman, G.A.; Fisman, P.H. Duration of therapeutic levels of intravitreally injected liposome-encapsulated clindamycin in the rabbit. Can. J. Ophthalmol. 1987, 22, 307–309. [Google Scholar]
- Wiechens, B.; Krausse, R.; Grammer, J.B.; Neumann, D.; Pleyer, U.; Duncker, G.I.W. Clearance of liposome-incorporated ciprofloxacin after intravitreal injection in rabbit eyes. Klin. Monatsbl. Augenheilk. 1998, 213, 28. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Velpandian, T.; Dhingra, N.; Jaiswal, J. Intravitreal Pharmacokinetics of Plain and Liposome-Entrapped Fluconazole in Rabbit Eyes. J. Ocul. Pharmacol. Therap. 2000, 16, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Claro, C.; Ruiz, R.; Cordero, E.; Pastor, M.T.; Lopez-Cortez, L.F.; Jimenez-Castellanos, M.R.; Lucero, M.J. Determination and pharmacokinetic profile of liposomal foscarnet in rabbit ocular tissues after intravitreal administration. Exp. Eye Res. 2008, 88, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Varschoclan, R.; Riazi-Esfahani, M.; Jeddi-Tehrani, M.; Mahmoudi, A.R.; Aghazadeh, S.; Mahbod, M.; Movassat, M.; Atyabi, F.; Sabzevari, A.; Dinarvand, R. Albuminated PLGA nanoparticles containing bevacizumab intended for ocular neovascularization treatment. J. Biomed. Mater. Res. 2015, 103, 3148–3156. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, E.; Ozeki, H.; Kunou, N.; Ogura, Y. Effect of Particle Size of Polymeric Nanospheres on Intravitreal Kinetics. Ophthal. Res. 2001, 33, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.D.; Khawaja, A.P.; Weizer, J.S. Glaucoma in Adults-Screening, Diagnosis, and Management: A Review. JAMA 2021, 325, 164–174. [Google Scholar] [CrossRef]
- Tavakoli, S.; Peynshaert, K.; Lajunen, T.; Devoldere, J.; del Amo, E.M.; Ruponen, M.; De Smedt, S.C.; Remaut, K.; Urtti, A. Ocular barriers to retinal delivery of intravitreal liposomes: Impact of vitreoretinal interface. J. Control. Rel. 2020, 328, 952–961. [Google Scholar] [CrossRef]
- Rimpelä, A.K.; Kiiski, I.; Deng, F.; Kidron, H.; Urtti, A. Pharmacokinetic Simulations of Intravitreal Biologicals: Aspects on Drug Delivery to the Posterior and Anterior Segments. Pharmaceutics 2018, 11, 9. [Google Scholar] [CrossRef] [Green Version]
- Kari, O.K.; Ndika, J.; Parkkila, P.; Louna, A.; Lajunen, T.; Puustinen, A.; Viitala, T.; Alenius, H.; Urtti, A. In situ analysis of liposome hard and soft protein corona structure and composition in a single label-free workflow. Nanoscale 2020, 12, 1728–1741. [Google Scholar] [CrossRef] [Green Version]
- Dickmann, L.; Yip, V.; Li, C.; Abundes, J.; Maia, M.; Young, C.; Stainton, S.; Hass, P.E.; Joseph, S.B.; Prabhu, S.; et al. Evaluation of Fluorophotometry to Assess the Vitreal Pharmacokinetics of Protein Therapeutics. Invest. Ophthalmol. Vis. Sci. 2015, 56, 6991–6999. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Formulation (Polymer) | Size ± SD (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|
| Neutral polymersome (PPP) | 95 ± 11 | 0.257 | 0.7 |
| Cationic polymersome (pPPP) | 115 ± 9 | 0.291 | +13.2 |
| Anionic polymersome (nPPP) | 89 ± 19 | 0.297 | −11.9 |
| Polymeric micelle (p22) | 31 ± 4 | 0.088 | −2.7 |
| Polymeric micelle (p42) | 43 ± 6 | 0.054 | −4.9 |
| Formulation (Polymer) | Dv (µm2/s) | Dw (µm2/s) | Dw/Dv |
|---|---|---|---|
| Neutral polymersome (PPP) | 0.40 ± 0.10 | 6.93 | 17.3 |
| Cationic polymersome (pPPP) | 0.33 ± 0.09 | 5.73 | 17.5 |
| Anionic polymersome (nPPP) | 0.48 ± 0.12 | 7.40 | 15.5 |
| Formulation | Rabbit | AUC (h mg/mL) | Half-Life (Days) | CL (µL/h) | Vss (mL) |
|---|---|---|---|---|---|
| Micelle | 22 | 91.9 | 5.8 | 5.4 | 1.09 |
| Micelle | 20 | 124.7 | 9.2 | 4.0 | 1.28 |
| Micelle | 17 | 137.8 | 9.5 | 3.6 | 1.20 |
| Micelle | 15 | 122.7 | 4.3 | 4.1 | 0.60 |
| Polymersome | 21 | 36.8 | 11.4 | 8.7 | 3.42 |
| Polymersome | 19 | 193.7 | 32.7 | 1.7 | 1.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junnuthula, V.; Sadeghi Boroujeni, A.; Cao, S.; Tavakoli, S.; Ridolfo, R.; Toropainen, E.; Ruponen, M.; van Hest, J.C.M.; Urtti, A. Intravitreal Polymeric Nanocarriers with Long Ocular Retention and Targeted Delivery to the Retina and Optic Nerve Head Region. Pharmaceutics 2021, 13, 445. https://doi.org/10.3390/pharmaceutics13040445
Junnuthula V, Sadeghi Boroujeni A, Cao S, Tavakoli S, Ridolfo R, Toropainen E, Ruponen M, van Hest JCM, Urtti A. Intravitreal Polymeric Nanocarriers with Long Ocular Retention and Targeted Delivery to the Retina and Optic Nerve Head Region. Pharmaceutics. 2021; 13(4):445. https://doi.org/10.3390/pharmaceutics13040445
Chicago/Turabian StyleJunnuthula, Vijayabhaskarreddy, Amir Sadeghi Boroujeni, Shoupeng Cao, Shirin Tavakoli, Roxane Ridolfo, Elisa Toropainen, Marika Ruponen, Jan C. M. van Hest, and Arto Urtti. 2021. "Intravitreal Polymeric Nanocarriers with Long Ocular Retention and Targeted Delivery to the Retina and Optic Nerve Head Region" Pharmaceutics 13, no. 4: 445. https://doi.org/10.3390/pharmaceutics13040445
APA StyleJunnuthula, V., Sadeghi Boroujeni, A., Cao, S., Tavakoli, S., Ridolfo, R., Toropainen, E., Ruponen, M., van Hest, J. C. M., & Urtti, A. (2021). Intravitreal Polymeric Nanocarriers with Long Ocular Retention and Targeted Delivery to the Retina and Optic Nerve Head Region. Pharmaceutics, 13(4), 445. https://doi.org/10.3390/pharmaceutics13040445