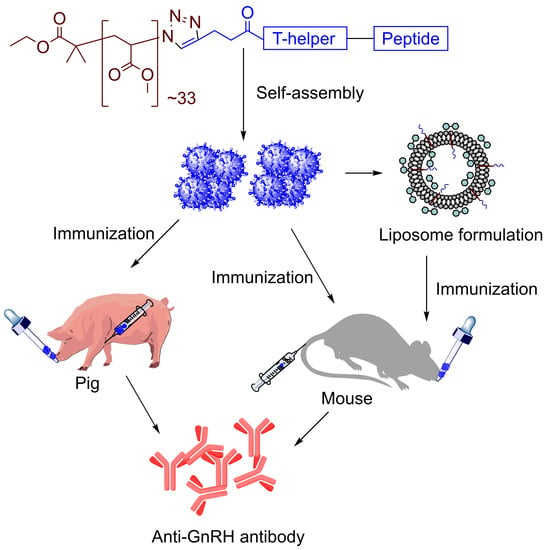
Polyacrylate-GnRH Peptide Conjugate as an Oral Contraceptive Vaccine Candidate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Synthesis of Peptides, PADRE-GnRH with Alkyne Moiety (P-GnRH)
2.4. Synthesis of Pig T helper-GnRH with Alkyne Moiety (PT-GnRH)
2.5. Synthesis of Polymer-Peptide Conjugates, 1 and 2
2.6. Preparation of 1-Tr
2.7. Preparation of Liposomes, L-0 and L-1
2.8. Preparation of L-1-Tr
2.9. Characterization of the Size, Zeta-Potential, and Morphology of the Vaccine Candidates
2.10. Subcutaneous Immunization of Mice
2.11. Oral Immunization of Mice
2.12. Intramuscular and Oral Immunization of Pigs
2.13. Determination of Antibody Titers (IgG) in Mice
2.14. Determination of IgG Antibody Titers in Pigs
3. Results
3.1. Synthesis and Characterization
3.2. Immunization Study in Mice
3.3. Immunization Study in Pigs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lefebvre, J.; Gauthier, G.; Giroux, J.-F.; Reed, A.; Reed, E.T.; Belanger, L. The greater snow goose Anser caerulescens atlanticus: Managing an overabundant population. Ambio 2017, 46, 262–274. [Google Scholar] [CrossRef] [Green Version]
- Payo-Payo, A.; Oro, D.; Igual, J.M.; Jover, L.; Sanpera, C.; Tavecchia, G. Population control of an overabundant species achieved through consecutive anthropogenic perturbations. Ecol. Appl. 2015, 25, 2228–2239. [Google Scholar] [CrossRef] [Green Version]
- Lueders, I.; Young, D.; Maree, L.; Van der Horst, G.; Luther, I.; Botha, S.; Tindall, B.; Fosgate, G.; Ganswindt, A.; Bertschinger, H.J. Effects of GnRH vaccination in wild and captive African elephant bulls (Loxodonta africana) on reproductive organs and semen quality. PLoS ONE 2017, 12, e0178270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somgird, C.; Homkong, P.; Sripiboon, S.; Brown, J.L.; Stout, T.A.E.; Colenbrander, B.; Mahasawangkul, S.; Thitaram, C. Potential of a gonadotropin-releasing hormone vaccine to suppress musth in captive male Asian elephants (Elephas maximus). Anim. Reprod. Sci. 2016, 164, 111–120. [Google Scholar] [CrossRef]
- Kustritz, M.V.R. Population control in small animals. Vet. Clin. Small Anim. Pract. 2018, 48, 721–732. [Google Scholar] [CrossRef]
- Miesner, M.D.; Anderson, D.E. Surgical management of common disorders of feedlot calves. Vet. Clin. Food Anim. Pract. 2015, 31, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Massei, G.; Cowan, D. Fertility control to mitigate human-wildlife conflicts: A review. Wildl. Res. 2014, 41, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, J.F.; Lyda, R.O.; Frank, K.M. Contraceptive vaccines for wildlife: A review. Am. J. Reprod. Immunol. 2011, 66, 40–50. [Google Scholar] [CrossRef]
- Naz, R.K.; Gupta, S.K.; Gupta, J.C.; Vyas, H.K.; Talwar, G.P. Recent advances in contraceptive vaccine development: A mini-review. Hum. Reprod. 2005, 20, 3271–3283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, M.E.; Thain, D.S.; Cameron, E.Z.; Miller, L.A. Multi-year fertility reduction in free-roaming feral horses with single-injection immunocontraceptive formulations. Wildl. Res. 2010, 37, 475–481. [Google Scholar] [CrossRef]
- Levy, J.K.; Friary, J.A.; Miller, L.A.; Tucker, S.J.; Fagerstone, K.A. Long-term fertility control in female cats with GonaCon™, a GnRH immunocontraceptive. Theriogenology 2011, 76, 1517–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millar, R.P. GnRHs and GnRH receptors. Anim. Reprod. Sci. 2005, 88, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Schally, A.V.; Arimura, A.; Baba, Y.; Nair, R.M.G.; Matsuo, H.; Redding, T.W.; Debeljuk, L.; White, W.F. Isolation and properties of the FSH and LH-releasing hormone. Biochem. Biophys. Res. Commun. 1971, 43, 393–399. [Google Scholar] [CrossRef]
- Casper, R.F. Clinical uses of gonadotropin-releasing hormone analogues. CMAJ Can. Med. Assoc. J. 1991, 144, 153. [Google Scholar]
- Bartfai, G. Clinical applications of gonadotrophin-releasing hormone and its analogues. Hum. Reprod. 1988, 3, 51–57. [Google Scholar] [CrossRef]
- Gionfriddo, J.P.; Denicola, A.J.; Miller, L.A.; Fagerstone, K.A. Efficacy of GnRH immunocontraception of wild whitetailed deer in New Jersey. Wildl. Soc. Bull. 2011, 35, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Massei, G.; Cowan, D.P.; Coats, J.; Bellamy, F.; Quy, R.; Pietravalle, S.; Brash, M.; Miller, L.A. Long-term effects of immunocontraception on wild boar fertility, physiology and behaviour. Wildl. Res. 2012, 39, 378–385. [Google Scholar] [CrossRef]
- Quy, R.J.; Massei, G.; Lambert, M.S.; Coats, J.; Miller, L.A.; Cowan, D.P. Effects of a GnRH vaccine on the movement and activity of free-living wild boar (Sus scrofa). Wildl. Res. 2014, 41, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Massei, G.; Koon, K.-K.; Law, S.-I.; Gomm, M.; Mora, D.S.O.; Callaby, R.; Palphramand, K.; Eckery, D.C. Fertility control for managing free-roaming feral cattle in Hong Kong. Vaccine 2018, 36, 7393–7398. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.A.; Fagerstone, K.A.; Eckery, D.C. Twenty years of immunocontraceptive research: Lessons learned. J. Zoo Wildl. Med. 2013, 44, S84–S96. [Google Scholar] [CrossRef] [Green Version]
- Fraker, M.A.; Brown, R.G.; Gaunt, G.E.; Kerr, J.A.; Pohajdak, B. Long-lasting, single-dose immunocontraception of feral fallow deer in British Columbia. J. Wildl. Manag. 2002, 66, 1141–1147. [Google Scholar] [CrossRef]
- Groenendaal, H.; Zagmutt, F.J.; Patton, E.A.; Wells, S.J. Cost-benefit analysis of vaccination against Mycobacterium avium ssp. paratuberculosis in dairy cattle, given its cross-reactivity with tuberculosis tests. J. Dairy Sci. 2015, 98, 6070–6084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, J.G.; Baker, D.L.; Davis, T.L.; Conner, M.M.; Lothridge, A.H.; Nett, T.M. Effects of Gonadotropin-Releasing Hormone Immunization on Reproductive Function and Behavior in Captive Female Rocky Mountain Elk (Cervus elaphus nelsoni). Biol. Reprod. 2011, 85, 1152–1160. [Google Scholar] [CrossRef] [Green Version]
- Skwarczynski, M.; Zaman, M.; Urbani, C.N.; Lin, I.-C.; Jia, Z.; Batzloff, M.R.; Good, M.F.; Monteiro, M.J.; Toth, I. Polyacrylate dendrimer nanoparticles: A selfadjuvanting vaccine delivery system. Angew. Chem. 2010, 122, 5878–5881. [Google Scholar] [CrossRef]
- Chandrudu, S.; Bartlett, S.; Khalil, Z.G.; Jia, Z.; Hussein, W.M.; Capon, R.J.; Batzloff, M.R.; Good, M.F.; Monteiro, M.J.; Skwarczynski, M.; et al. Linear and branched polyacrylates as a delivery platform for peptide-based vaccines. Ther. Deliv. 2016, 7, 601–609. [Google Scholar] [CrossRef]
- Ahmad Fuaad, A.A.H.; Jia, Z.; Zaman, M.; Hartas, J.; Ziora, Z.M.; Lin, I.-C.; Moyle, P.M.; Batzloff, M.R.; Good, M.F.; Monteiro, M.J.; et al. Polymer–peptide hybrids as a highly immunogenic single-dose nanovaccine. Nanomedicine 2014, 9, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Nevagi, R.J.; Skwarczynski, M.; Toth, I. Polymers for Subunit Vaccine Delivery. Eur. Polym. J. 2019, 114, 397–410. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Kumar Giddam, A.; Hussein, W.M.; Jia, Z.; McMillan, N.A.J.; Monteiro, M.J.; Toth, I.; Skwarczynski, M. Self-adjuvanting therapeutic peptide-based vaccine induce CD8+ cytotoxic T lymphocyte responses in a murine human papillomavirus tumor model. Curr. Drug Deliv. 2015, 12, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khongkow, M.; Liu, T.-Y.; Bartlett, S.; Hussein, W.M.; Nevagi, R.; Jia, Z.F.; Monteiro, M.J.; Wells, J.W.; Ruktanonchai, U.R.; Skwarczynski, M.; et al. Liposomal formulation of polyacrylate-peptide conjugate as a new vaccine candidate against cervical cancer. Precis. Nanomed. 2018, 1, 183–193. [Google Scholar]
- Faruck, M.O.; Zhao, L.; Hussein, W.M.; Khalil, Z.G.; Capon, R.J.; Skwarczynski, M.; Toth, I. Polyacrylate-Peptide Antigen Conjugate as a Single-Dose Oral Vaccine against Group A Streptococcus. Vaccines 2020, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Fuaad, A.A.H.A.; Skwarczynski, M.; Toth, I. The Use of Microwave-Assisted Solid-Phase Peptide Synthesis and Click Chemistry for the Synthesis of Vaccine Candidates Against Hookworm Infection. In Vaccine Design; Springer: New York, NY, USA, 2016; pp. 639–653. [Google Scholar]
- Dai, C.; Stephenson, R.J.; Skwarczynski, M.; Toth, I. Application of Fmoc-SPPS, Thiol-Maleimide Conjugation, and Copper(I)-Catalyzed Alkyne-Azide Cycloaddition “Click” Reaction in the Synthesis of a Complex. Peptide-Based Vaccine Candidate Against Group A Streptococcus. In Methods in Molecular Biology; Clifton, N.J., Ed.; Humana: New York, NY, USA, 2020; pp. 13–27. [Google Scholar]
- Hussein, W.M.; Liu, T.-Y.; Jia, Z.; McMillan, N.A.J.; Monteiro, M.J.; Toth, I.; Skwarczynski, M. Multiantigenic peptide-polymer conjugates as therapeutic vaccines against cervical cancer. Bioorg. Med. Chem. 2016, 24, 4372–4380. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-Y.; Hussein, W.M.; Jia, Z.; Ziora, Z.M.; McMillan, N.A.J.; Monteiro, M.J.; Toth, I.; Skwarczynski, M. Self-adjuvanting polymer–peptide conjugates as therapeutic vaccine candidates against cervical cancer. Biomacromolecules 2013, 14, 2798–2806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaffar, K.A.; Marasini, N.; Giddam, A.K.; Batzloff, M.R.; Good, M.F.; Skwarczynski, M.; Toth, I. The Role of Size in Development of Mucosal Liposome-Lipopeptide Vaccine Candidates against Group A Streptococcus. Med. Chem. 2017, 13, 22–27. [Google Scholar] [CrossRef]
- Ghaffar, K.A.; Marasini, N.; Giddam, A.K.; Batzloff, M.R.; Good, M.F.; Skwarczynski, M.; Toth, I. Liposome-based intranasal delivery of lipopeptide vaccine candidates against group A streptococcus. Acta Biomater. 2016, 41, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-Y.; Hussein, W.M.; Giddam, A.K.; Jia, Z.; Reiman, J.M.; Zaman, M.; McMillan, N.A.J.; Good, M.F.; Monteiro, M.J.; Toth, I.; et al. Polyacrylate-based delivery system for self-adjuvanting anticancer peptide vaccine. J. Med. Chem. 2015, 58, 888–896. [Google Scholar] [CrossRef] [Green Version]
- Zaman, M.; Skwarczynski, M.; Malcolm, J.M.; Urbani, C.N.; Jia, Z.; Batzloff, M.R.; Good, M.F.; Monteiro, M.J.; Toth, I. Self-adjuvanting polyacrylic nanoparticulate delivery system for Group A streptococcus (GAS) vaccine. Nanomed. Nanotech. Biol. Med. 2011, 7, 168–173. [Google Scholar] [CrossRef]
- Ebner, F.; Schwiertz, P.; Steinfelder, S.; Pieper, R.; Zentek, J.; Schutze, N.; Baums, C.G.; Alber, G.; Geldhof, P.; Hartmann, S. Pathogen-reactive T helper cell analysis in the pig. Front. Immunol. 2017, 8, 565. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, D.; Varamini, P.; Simerska, P.; Michael, J.D.; Toth, I. Design, synthesis and evaluation of a gonadotropin releasing hormone-based subunit vaccine in rams (Ovis aries). Vaccine 2015, 33, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, D.; Simerska, P.; Chang, C.-H.; Mansfeld, F.M.; Varamini, P.; Michael, J.D.; Toth, I. Active immunisation of mice with GnRH lipopeptide vaccine candidates: Importance of T helper or multi-dimer GnRH epitope. Bioorganic Med. Chem. 2014, 22, 4848–4854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyle, P.M.; Toth, I. Modern Subunit Vaccines: Development, Components, and Research Opportunities. ChemMedChem 2013, 8, 360–376. [Google Scholar] [CrossRef]
- Alexander, J.; del Guercio, M.-F.; Maewal, A.; Qiao, L.; Fikes, J.; Chesnut, R.W.; Paulson, J.; Bundle, D.R.; DeFrees, S.; Sette, A. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J. Immunol. 2000, 164, 1625–1633. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, L.; Tian, C.; Zhang, G.; Huo, G.; Tang, L.; Li, Y. Immunogenicity of recombinant classic swine fever virus CD8+ T lymphocyte epitope and porcine parvovirus VP2 antigen coexpressed by Lactobacillus casei in swine via oral vaccination. Clin. Vaccine Immunol. 2011, 18, 1979–1986. [Google Scholar] [CrossRef] [Green Version]
- Tarradas, J.; Monso, M.; Munoz, M.; Rosell, R.; Fraile, L.; Frias, M.T.; Domingo, M.; Andreu, D.; Sobrino, F.; Ganges, L. Partial protection against classical swine fever virus elicited by dendrimeric vaccine-candidate peptides in domestic pigs. Vaccine 2011, 29, 4422–4429. [Google Scholar] [CrossRef]
- Pauly, T.; Elbers, K.; Konig, M.; Lengsfeld, T.; Saalmuller, A.; Thiel, H.-J. Classical swine fever virus-specific cytotoxic T lymphocytes and identification of a T cell epitope. J. Gen. Virol. 1995, 76, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Armengol, E.; Wiesmuller, K.-H.; Wienhold, D.; Buttner, M.; Pfaff, E.; Jung, G.; Saalmuller, A. Identification of T-cell epitopes in the structural and non-structural proteins of classical swine fever virus. J. Gen. Virol. 2002, 83, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarradas, J.; Monso, M.; Fraile, L.; Beatriz, G.; Munoz, M.; Rosell, R.; Riquelme, C.; Perez, L.J.; Nofrarias, M.; Domingo, M.; et al. A T-cell epitope on NS3 non-structural protein enhances the B and T cell responses elicited by dendrimeric constructions against CSFV in domestic pigs. Vet. Immunol. Immunopathol. 2012, 150, 36–46. [Google Scholar] [CrossRef]
- Liao, Y.-C.; Lin, H.H.; Lin, C.H.; Chung, W.B. Identification of Cytotoxic T Lymphocyte Epitopes on Swine Viruses: Multi-Epitope Design for Universal T Cell Vaccine. PLoS ONE 2013, 8, e84443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Qian, P.; Liu, L.; Qian, S.; Chen, H.; Li, X. Virus-like particles of chimeric recombinant porcine circovirus type 2 as antigen vehicle carrying foreign epitopes. Viruses 2014, 6, 4839–4855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Li, J.; Bi, Y.; Yang, L.; Meng, S.; Zhou, Y.; Jia, X.; Meng, S.; Sun, L.; Liu, W. Synthetic B- and T-cell epitope peptides of porcine reproductive and respiratory syndrome virus with Gp96 as adjuvant induced humoral and cell-mediated immunity. Vaccine 2013, 31, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Burmakina, G.; Malogolovkin, A.; Tulman, E.R.; Xu, W.; Delhon, G.; Kolbasov, D.; Rock, D.L. Identification of T-cell epitopes in African swine fever virus CD2v and C-type lectin proteins. J. Gen. Virol. 2019, 100, 259–265. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, J.; Nahar, U.J.; Khalil, Z.G.; Capon, R.J.; Hussein, W.M.; Skwarczynski, M.; Toth, I. A dual-adjuvanting strategy for peptide-based subunit vaccines against group A Streptococcus: Lipidation and polyelectrolyte complexes. Bioorganic Med. Chem. 2020, 28, 115823. [Google Scholar] [CrossRef]
- Azmi, F.; Ahmad Fuaad, A.A.H.; Skwarczynski, M.; Toth, I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum. Vaccines Immunother. 2014, 10, 778–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, J.R.; Haughney, S.L.; Mallapragada, S.K. Effective polymer adjuvants for sustained delivery of protein subunit vaccines. Acta Biomater. 2015, 14, 104–114. [Google Scholar] [CrossRef]
- Sahdev, P.; Ochyl, L.J.; Moon, J.J. Biomaterials for nanoparticle vaccine delivery systems. Pharm. Res. 2014, 31, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Simerska, P.; Toth, I. Synthetic polyacrylate polymers as particulate intranasal vaccine delivery systems for the induction of mucosal immune response. Curr. Drug Deliv. 2010, 7, 118–124. [Google Scholar] [CrossRef]
- Bartlett, S.; Skwarczynski, M.; Xie, X.; Toth, I.; Loukas, A.; Eichenberger, R.M. Development of natural and unnatural amino acid delivery systems against hookworm infection. Precis. Nanomed. 2020, 3, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, S.; Eichenberger, R.M.; Nevagi, R.J.; Ghaffar, K.A.; Marasini, N.; Dai, Y.; Loukas, A.; Toth, I.; Skwarczynski, M. Lipopeptide-Based Oral Vaccine Against Hookworm Infection. J. Infect. Dis. 2020, 221, 934–942. [Google Scholar] [CrossRef]
- Marasini, N.; Skwarczynski, M.; Toth, I. Oral delivery of nanoparticle-based vaccines. Expert Rev. Vaccines 2014, 13, 1361–1376. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Bhatia, E.; Sharma, S.; Ahamad, N.; Banerjee, R. Advancements in prophylactic and therapeutic nanovaccines. Acta Biomater. 2020, 108, 1–21. [Google Scholar] [CrossRef]
- Crowe, J.H.; Hoekstra, F.A.; Crowe, L.M. Anhydrobiosis. Annu. Rev. Physiol. 1992, 54, 579–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Oldenhof, H.; Sydykov, B.; Bigalk, J.; Sieme, H.; Wolkers, W.F. Freeze-drying of mammalian cells using trehalose: Preservation of DNA integrity. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Ghaffar, K.A.; Kumar Giddam, A.; Zaman, M.; Skwarczynski, M.; Toth, I. Liposomes as nanovaccine delivery systems. Curr. Top. Med. Chem. 2014, 14, 1194–1208. [Google Scholar] [CrossRef]
- Shah, R.R.; O’Hagan, D.T.; Amiji, M.M.; Brito, L.A. The impact of size on particulate vaccine adjuvants. Nanomedicine 2014, 9, 2671–2681. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Toth, I. Recent advances in peptide-based subunit nanovaccines. Nanomedicine 2014, 9, 2657–2669. [Google Scholar] [CrossRef]
- Stratmann, T. Cholera toxin subunit B as adjuvant—An accelerator in protective immunity and a break in autoimmunity. Vaccines 2015, 3, 579–596. [Google Scholar] [CrossRef] [Green Version]
- George Chandy, A.; Hultkrantz, S.; Raghavan, S.; Czerkinsky, C.; Lebens, M.; Telemo, E.; Holmgren, J. Oral tolerance induction by mucosal administration of cholera toxin Bcoupled antigen involves Tcell proliferation in vivo and is not affected by depletion of CD25+ T cells. Immunology 2006, 118, 311–320. [Google Scholar] [CrossRef]
- Sun, J.-B.; Flach, C.-F.; Czerkinsky, C.; Holmgren, J. B lymphocytes promote expansion of regulatory T cells in oral tolerance: Powerful induction by antigen coupled to cholera toxin B subunit. J. Immunol. 2008, 181, 8278–8287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauer, A.; Lange, E.; Kaden, V. Oral immunisation of wild boar against classical swine fever: Uptake studies of new baits and investigations on the stability of lyophilised C-strain vaccine. Eur. J. Wildl. Res. 2006, 52, 271–276. [Google Scholar] [CrossRef]
- Rossi, S.; Staubach, C.; Blome, S.; Guberti, V.; Thulke, H.-H.; Vos, A.; Koenen, F.; Le Potier, M.-F. Controlling of CSFV in European wild boar using oral vaccination: A review. Front. Microbiol. 2015, 6, 1141. [Google Scholar] [CrossRef]
- Calenge, C.; Rossi, S. Bayesian modelling of hunting data may improve the understanding of host-parasite systems: Wild boar diseases and vaccination as an example. J. Theor. Biol. 2014, 343, 32–43. [Google Scholar] [CrossRef]
- Feliziani, F.; Blome, S.; Petrini, S.; Giammarioli, M.; Iscaro, C.; Severi, G.; Convito, L.; Pietschmann, J.; Beer, M.; De Mia, G.M. First assessment of classical swine fever marker vaccine candidate CP7_E2alf for oral immunization of wild boar under field conditions. Vaccine 2014, 32, 2050–2055. [Google Scholar] [CrossRef]
- Moennig, V. The control of classical swine fever in wild boar. Front. Microbiol. 2015, 6, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, S.; Pol, F.; Forot, B.; Masse-Provin, N.; Rigaux, S.; Bronner, A.; Le Potier, M.-F. Preventive vaccination contributes to control classical swine fever in wild boar (Sus scrofa sp.). Vet. Microbiol. 2010, 142, 99–107. [Google Scholar] [CrossRef]
- Barasona, J.A.; Gallardo, C.; Cadenas-Fernandez, E.; Jurado, C.; Rivera, B.; Rodriguez-Bertos, A.; Arias, M.; Sanchez-Vizcaino, J.M. First Oral Vaccination of Eurasian Wild Boar Against African Swine Fever Virus Genotype II. Front. Vet. Sci. 2019, 6, 137. [Google Scholar] [CrossRef]
- Bosch-Camós, L.; López, E.; Rodriguez, F. African swine fever vaccines: A promising work still in progress. Porc. Health Manag. 2020, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-K.; Kim, H.-H.; Choi, S.-S.; Lee, S.H.; Cho, I.-S. A recombinant rabies virus (ERAGS) for use in a bait vaccine for swine. Clin. Exp. Vaccine Res. 2016, 5, 169–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunius, C.; Zamaratskaia, G.; Andersson, K.; Chen, G.; Norrby, M.; Madej, A.; Lundstrom, K. Early immunocastration of male pigs with Improvac®—Effect on boar taint, hormones and reproductive organs. Vaccine 2011, 29, 9514–9520. [Google Scholar] [CrossRef]
- Oliviero, C.; Lindh, L.; Peltoniemi, O. BOARD INVITED REVIEW: Immunocontraception as a possible tool to reduce feral pig populations: Recent and future perspectives. J. Anim. Sci. 2019, 97, 2283–2290. [Google Scholar] [CrossRef]
- Albrecht, A.K. Review on the consequences of using Improvac TM in modern pig production. Vet. Sci. Dev. 2013, 3, e1. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Toth, I. Non-invasive mucosal vaccine delivery: Advantages, challenges and the future. Expert Opin. Drug Deliv. 2020, 17, 435–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballesteros, C.; Gortazar, C.; Canales, M.; Vicente, J.; Lasagna, A.; Gamarra, J.A.; Carrasco-Garcia, R.; De la Fuente, J. Evaluation of baits for oral vaccination of European wild boar piglets. Res. Vet. Sci. 2009, 86, 388–393. [Google Scholar] [CrossRef] [PubMed]





| Compound | Particle Size (nm) | Polydispersity Index (PDI) |
|---|---|---|
| 1 | 62 ± 1 | 0.36 ± 0.03 |
| 1-Tr | 140 ± 4 | 0.19 ± 0.07 |
| L-0 | 99 ± 1 | 0.14 ± 0.03 |
| L-1 | 146 ± 2 | 0.19 ± 0.04 |
| L-1-Tr | 171 ± 2 | 0.22 ± 0.09 |
| 2 | 141 ± 5 | 0.08 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faruck, M.O.; Koirala, P.; Yang, J.; D’Occhio, M.J.; Skwarczynski, M.; Toth, I. Polyacrylate-GnRH Peptide Conjugate as an Oral Contraceptive Vaccine Candidate. Pharmaceutics 2021, 13, 1081. https://doi.org/10.3390/pharmaceutics13071081
Faruck MO, Koirala P, Yang J, D’Occhio MJ, Skwarczynski M, Toth I. Polyacrylate-GnRH Peptide Conjugate as an Oral Contraceptive Vaccine Candidate. Pharmaceutics. 2021; 13(7):1081. https://doi.org/10.3390/pharmaceutics13071081
Chicago/Turabian StyleFaruck, Mohammad O., Prashamsa Koirala, Jieru Yang, Michael J. D’Occhio, Mariusz Skwarczynski, and Istvan Toth. 2021. "Polyacrylate-GnRH Peptide Conjugate as an Oral Contraceptive Vaccine Candidate" Pharmaceutics 13, no. 7: 1081. https://doi.org/10.3390/pharmaceutics13071081
APA StyleFaruck, M. O., Koirala, P., Yang, J., D’Occhio, M. J., Skwarczynski, M., & Toth, I. (2021). Polyacrylate-GnRH Peptide Conjugate as an Oral Contraceptive Vaccine Candidate. Pharmaceutics, 13(7), 1081. https://doi.org/10.3390/pharmaceutics13071081