Synthesis and Properties of Targeted Radioisotope Carriers Based on Poly(Acrylic Acid) Nanogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
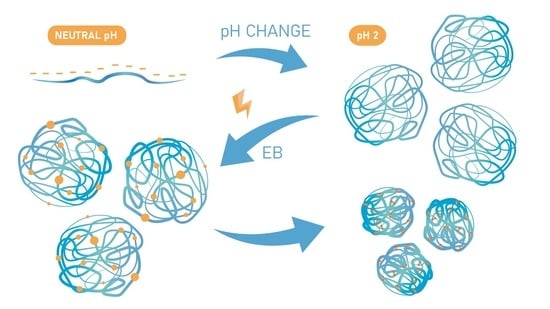
2.2. Radiation-Induced Synthesis of Nanogels
2.3. Nanogel Coupling with a Model Compound
2.4. In-House Synthesis of Custom Bombesin Derivative
2.4.1. Rink Amide Resin Loading
2.4.2. Deprotection
2.4.3. Standard Coupling Procedure
2.4.4. 2-Chlorotrityl Chloride Resin Loading
2.4.5. Deprotection
2.4.6. Coupling of Tri-tert-butyl 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate to Unprotected Lysine
2.4.7. Cleavage from the 2-Chlorotritylchloride Resin
2.4.8. Coupling of Fmoc-Lys(DOTA-tri-t-Bu-ester)-OH to Peptide on Rink Amide Resin
2.4.9. Coupling of Fmoc-Gln(Trt)-OH and Fmoc-Lys(Boc)-OH
2.4.10. Cleavage from the Rink Amide Resin
2.4.11. Synthesis of Bombesin Derivative without DOTA
2.5. Nanogel Coupling with Bombesin Derivative
2.6. Purification of Nanogels Coupled with Model Compound/Oligopeptide
2.7. Characterization of Nanogels
2.7.1. Static Light-Scattering Measurements (SLS)
2.7.2. Dynamic Light-Scattering Measurements (DLS)
2.7.3. FTIR Infrared Spectroscopy
2.7.4. UV-Vis Spectroscopy
2.7.5. Fluorescence Spectroscopy
2.7.6. 1H NMR Nuclear Magnetic Resonance Spectroscopy
2.8. Radiolabeling of DOTA-Bombesin Derivative and Quality Control
3. Results and Discussion
3.1. Radiation-Induced Synthesis of Nanogels
3.2. Coupling PAA Nanogels with a Simple Amine as Model Compound for Oligopeptide
3.3. Coupling PAA Nanogels with Oligopeptide
3.4. Radiolabeling of DOTA-Bombesin Derivative
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, D.K.; Balekar, N.; Mishra, P.K. Nanoengineered strategies for siRNA delivery: From target assessment to cancer therapeutic efficacy. Drug Deliv. Transl. Res. 2017, 7, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef]
- Ocampo-García, B.; Gibbens-Bandala, B.; Morales-Avila, E.; Melendez-Alafort, L.; Khoobchandani, M.; Trujillo-Nolasco, M.; Katti, K. V Dual-targeted therapy and molecular imaging with radiolabeled nanoparticles. In Biotechnology Products in Everyday Life; Khoobchandani, M., Saxena, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 201–219. ISBN 978-3-319-92399-4. [Google Scholar]
- Bansal, R.; Nagórniewicz, B.; Prakash, J. Clinical advancements in the targeted therapies against liver fibrosis. Mediat. Inflamm. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S. Radionanomedicine: Combined Nuclear and Nanomedicine, 1st ed.; Lee, D.S., Ed.; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-67719-4. [Google Scholar]
- Alemán, J.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabatino, M.A.; Ditta, L.A.; Conigliaro, A.; Dispenza, C. A multifuctional nanoplatform for drug targeted delivery based on radiation-engineered nanogels. Radiat. Phys. Chem. 2018, 169, 108059. [Google Scholar] [CrossRef]
- Argentiere, S.; Blasi, L.; Ciccarella, G.; Barbarella, G.; Cingolani, R.; Gigli, G. Synthesis of poly(acrylic acid) nanogels and application in loading and release of an oligothiophene fluorophore and its bovine serum albumin conjugate. Macromol. Symp. 2009, 281, 69–76. [Google Scholar] [CrossRef]
- Argentiere, S.; Blasi, L.; Morello, G.; Gigli, G. A novel pH-responsive nanogel for the controlled uptake and release of hydrophobic and cationic solutes. J. Phys. Chem. C 2011, 115, 16347–16353. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chemie Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [Green Version]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Dispenza, C.; Spadaro, G.; Jonsson, M. Radiation engineering of multifunctional nanogels. Top. Curr. Chem. 2016, 374, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.J.; Thulasidasan, A.K.T.; Anto, R.J.; Chithralekha, D.N.; Narayanan, A.; Kumar, G.S.V. Folic acid conjugated cross-linked acrylic polymer (FA-CLAP) hydrogel for site specific delivery of hydrophobic drugs to cancer cells. J. Nanobiotechnol. 2014, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kulhari, H.; Pooja, D.; Singh, M.K.; Kuncha, M.; Adams, D.J.; Sistla, R. Bombesin-conjugated nanoparticles improve the cytotoxic efficacy of docetaxel against gastrin-releasing but androgen-independent prostate cancer. Nanomedicine 2015, 10, 2847–2859. [Google Scholar] [CrossRef] [PubMed]
- Adamo, G.; Grimaldi, N.; Campora, S.; Bulone, D.; Bondì, M.L.; Al-Sheikhly, M.; Sabatino, M.A.; Dispenza, C.; Ghersi, G. Multi-functional nanogels for tumor targeting and redox-sensitive drug and siRNA delivery. Molecules 2016, 21, 1594. [Google Scholar] [CrossRef]
- Zhang, X.; Malhotra, S.; Molina, M.; Haag, R. Micro- and nanogels with labile crosslinks-from synthesis to biomedical applications. Chem. Soc. Rev. 2015, 44, 1948–1973. [Google Scholar] [CrossRef] [Green Version]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadlubowski, S.; Ulanski, P.; Rosiak, J.M. Synthesis of tailored nanogels by means of two-stage irradiation. Polymer 2012, 53, 1985–1991. [Google Scholar] [CrossRef]
- Henke, A.; Kadlubowski, S.; Ulanski, P.; Rosiak, J.M.; Arndt, K.-F. Radiation-induced cross-linking of polyvinylpyrrolidone-poly(acrylic acid) complexes. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2005, 236, 391–398. [Google Scholar] [CrossRef]
- Schmidt, T.; Janik, I.; Kadlubowski, S.; Ulanski, P.; Rosiak, J.M.; Reichelt, R.; Arndt, K.-F. Pulsed electron beam irradiation of dilute aqueous poly(vinyl methyl ether) solutions. Polymer 2005, 46, 9908–9918. [Google Scholar] [CrossRef]
- Kadlubowski, S.; Grobelny, J.; Olejniczak, W.; Cichomski, M.; Ulanski, P. Pulses of fast electrons as a tool to synthesize poly(acrylic acid) nanogels. Intramolecular cross-linking of linear polymer chains in additive-free aqueous solution. Macromolecules 2003, 36, 2484–2492. [Google Scholar] [CrossRef]
- Ulanski, P.; Rosiak, J.M. The use of radiation technique in the synthesis of polymeric nanogels. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1999, 151, 356–360. [Google Scholar] [CrossRef]
- Ulanski, P.; Janik, I.; Rosiak, J.M. Radiation formation of polymeric nanogels. Radiat. Phys. Chem. 1998, 52, 289–294. [Google Scholar] [CrossRef]
- Matusiak, M.; Kadlubowski, S.; Ulanski, P. Radiation-induced synthesis of poly(acrylic acid) nanogels. Radiat. Phys. Chem. 2018, 142, 125–129. [Google Scholar] [CrossRef]
- Ulanski, P.; Kadlubowski, S.; Rosiak, J.M. Synthesis of poly(acrylic acid) nanogels by preparative pulse radiolysis. Radiat. Phys. Chem. 2002, 63, 533–537. [Google Scholar] [CrossRef]
- Munavirov, B.; Gnezdilov, O.; Rudakova, M.; Antzutkin, O.N.; Filippov, A. Interaction of polyacrylic acid with lipid bilayers: Effect of polymer mass. Magn. Reson. Chem. 2013, 51, 750–755. [Google Scholar] [CrossRef]
- Munavirov, B.V.; Filippov, A.V.; Rudakova, M.A.; Antzutkin, O.N. Polyacrylic acid modifies local and lateral mobilities in lipid membranes. J. Dispers. Sci. Technol. 2014, 35, 848–858. [Google Scholar] [CrossRef]
- Yessine, M.-A.; Leroux, J.-C. Membrane-destabilizing polyanions: Interaction with lipid bilayers and endosomal escape of biomacromolecules. Adv. Drug Deliv. Rev. 2004, 56, 999–1021. [Google Scholar] [CrossRef]
- Fujiwara, M.; Grubbs, R.H.; Baldeschwieler, J.D. Characterization of pH-dependent poly(acrylic acid) complexation with phospholipid vesicles. J. Colloid Interface Sci. 1997, 185, 210–216. [Google Scholar] [CrossRef]
- Chieng, Y.Y.; Chen, S.B. Interaction between poly(acrylic acid) and phospholipid vesicles: Effect of pH, concentration, and molecular weight. J. Phys. Chem. B 2010, 114, 4828–4835. [Google Scholar] [CrossRef]
- Adamo, G.; Grimaldi, N.; Campora, S.; Sabatino, M.A.; Dispenza, C.; Ghersi, G. Glutathione-sensitive nanogels for drug release. Chem. Eng. Trans. 2014, 38, 457–462. [Google Scholar] [CrossRef]
- Dispenza, C.; Adamo, G.; Sabatino, M.A.; Grimaldi, N.; Bulone, D.; Bondì, M.L.; Rigogliuso, S.; Ghersi, G. Oligonucleotides-decorated-poly(N-vinyl pyrrolidone) nanogels for gene delivery. J. Appl. Polym. Sci. 2014, 131, 1–8. [Google Scholar] [CrossRef]
- Picone, P.; Ditta, L.A.; Sabatino, M.A.; Militello, V.; San Biagio, P.L.; Di Giacinto, M.L.; Cristaldi, L.; Nuzzo, D.; Dispenza, C.; Giacomazza, D.; et al. Ionizing radiation-engineered nanogels as insulin nanocarriers for the development of a new strategy for the treatment of Alzheimer’s disease. Biomaterials 2016, 80, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Sabatino, M.A.; Ditta, L.A.; Amato, A.; San Biagio, P.L.; Mulè, F.; Giacomazza, D.; Dispenza, C.; Di Carlo, M. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J. Control. Release 2018, 270, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Jon, S.; Khademhosseini, A.; Tran, T.N.T.; LaVan, D.A.; Langer, R. Nanoparticle-aptamer bioconjugates: A new approach for targeting prostate cancer cells. Cancer Res. 2004, 64, 7668–7672. [Google Scholar] [CrossRef] [Green Version]
- Mardhian, D.F.; Storm, G.; Bansal, R.; Prakash, J. Nano-targeted relaxin impairs fibrosis and tumor growth in pancreatic cancer and improves the efficacy of gemcitabine in vivo. J. Control. Release 2018, 290, 1–10. [Google Scholar] [CrossRef]
- Kang, T.; Gao, X.; Hu, Q.; Jiang, D.; Feng, X.; Zhang, X.; Song, Q.; Yao, L.; Huang, M.; Jiang, X.; et al. INGR-modified PEG-PLGA nanoparticles that recognize tumor vasculature and penetrate gliomas. Biomaterials 2014, 35, 4319–4332. [Google Scholar] [CrossRef]
- Chen, W.; Zou, Y.; Zhong, Z.; Haag, R. Cyclo(RGD)-decorated reduction-responsive nanogels mediate targeted chemotherapy of integrin overexpressing human glioblastoma in vivo. Small 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Cao, Z.; Yu, Q.; Xue, H.; Cheng, G.; Jiang, S. Nanoparticles for drug delivery prepared from amphiphilic PLGA zwitterionic block copolymers with sharp contrast in polarity between two blocks. Angew. Chemie Int. Ed. 2010, 49, 3771–3776. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Inoue, S.; Ljubimov, A.V.; Patil, R.; Portilla-Arias, J.; Hu, J.; Konda, B.; Wawrowsky, K.A.; Fujita, M.; Karabalin, N.; et al. Inhibition of brain tumor growth by intravenous poly (β-L-malic acid) nanobioconjugate with pH-dependent drug release. Proc. Natl. Acad. Sci. USA 2010, 107, 18143–18148. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Senanayake, T.H.; Warren, G.; Vinogradov, S.V. Hyaluronic acid-based nanogel-drug conjugates with enhanced anticancer activity designed for the targeting of CD44-positive and drug-resistant tumors. Bioconjug. Chem. 2013, 24, 658–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Ouyang, J.; Chen, Q.; Deng, C.; Meng, F.; Zhang, J.; Cheng, R.; Lan, Q.; Zhong, Z. EGFR and CD44 dual-targeted multifunctional hyaluronic acid nanogels boost protein delivery to ovarian and breast cancers in vitro and in vivo. ACS Appl. Mater. Interfaces 2017, 9, 24140–24147. [Google Scholar] [CrossRef]
- Irby, D.; Du, C.; Li, F.; States, U. Lipid–drug conjugate for enhancing drug delivery. Mol. Pharmacol. 2018, 14, 1325–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, L.; Du, Y.; Ismail, M.; He, R.; Hou, Y.; Fu, Z.; Zhang, Y.; Yao, C.; Li, X. Self-assembled liposomes of dual paclitaxel-phospholipid prodrug for anticancer therapy. Int. J. Pharm. 2017, 526, 11–22. [Google Scholar] [CrossRef]
- Hermanson, G.T. Chapter 14. Microparticles and nanoparticles. In Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013; pp. 549–587. ISBN 978-0-12-382239-0. [Google Scholar]
- Hermanson, G.T. Chapter 3. The reactions of bioconjugation. In Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013; pp. 664–668. ISBN 978-0-12-382239-0. [Google Scholar]
- Kunishima, M.; Kawachi, C.; Morita, J.; Terao, K.; Iwasaki, F.; Tani, S. 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride: An efficient condensing agent leading to the formation of amides and esters. Tetrahedron 1999, 55, 13159–13170. [Google Scholar] [CrossRef]
- D’Este, M.; Eglin, D.; Alini, M. A systematic analysis of DMTMM vs EDC/NHS for ligation of amines to hyaluronan in water. Carbohydr. Polym. 2014, 108, 239–246. [Google Scholar] [CrossRef]
- Kaminski, Z.J.; Kolesinska, B.; Kolesinska, J.; Sabatino, G.; Chelli, M.; Rovero, P.; Blaszczyk, M.; Glowka, M.L.; Papini, A.M. N-triazinylammonium tetrafluoroborates. A new generation of efficient coupling reagents useful for peptide synthesis. J. Am. Chem. Soc. 2005, 127, 16912–16920. [Google Scholar] [CrossRef]
- Kolesinska, B.; Rozniakowski, K.K.; Fraczyk, J.; Relich, I.; Maria Papini, A.; Kaminski, Z.J. The effect of counterion and tertiary amine on the efficiency of N-triazinylammonium sulfonates in solution and solid-phase peptide synthesis. Eur. J. Org. Chem. 2015, 2015, 401–408. [Google Scholar] [CrossRef]
- Palao-Suay, R.; Martín-Saavedra, F.M.; Rosa Aguilar, M.; Escudero-Duch, C.; Martín-Saldaña, S.; Parra-Ruiz, F.J.; Rohner, N.A.; Thomas, S.N.; Vilaboa, N.; San Román, J. Photothermal and photodynamic activity of polymeric nanoparticles based on α-tocopheryl succinate-RAFT block copolymers conjugated to IR-780. Acta Biomater. 2017, 57, 70–84. [Google Scholar] [CrossRef]
- Chai, H.J.; Kiew, L.V.; Chin, Y.; Norazit, A.; Noor, S.M.; Lo, Y.L.; Looi, C.Y.; Lau, Y.S.; Lim, T.M.; Wong, W.F.; et al. Renal targeting potential of a polymeric drug carrier, poly-L-glutamic acid, in normal and diabetic rats. Int. J. Nanomed. 2017, 12, 577–591. [Google Scholar] [CrossRef] [Green Version]
- Conejos-Sánchez, I.; Cardoso, I.; Oteo-Vives, M.; Romero-Sanz, E.; Paul, A.; Sauri, A.R.; Morcillo, M.A.; Saraiva, M.J.; Vicent, M.J. Polymer-doxycycline conjugates as fibril disrupters: An approach towards the treatment of a rare amyloidotic disease. J. Control. Release 2015, 198, 80–90. [Google Scholar] [CrossRef]
- Carlson, R.H. Radioimmunotherapy-Chemotherapy Targets Advanced Pancreatic Cancer. Oncol. Times 2014, 36, 54–55. [Google Scholar] [CrossRef]
- Definition of yttrium Y90 tacatuzumab tetraxetan. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/yttrium-y-90-tacatuzumab-tetraxetan (accessed on 28 July 2021).
- Breeman, W.A.P.; de Blois, E.; Sze Chan, H.; Konijnenberg, M.; Kwekkeboom, D.J.; Krenning, E.P. 68Ga-labeled DOTA-Peptides and 68Ga-labeled Radiopharmaceuticals for Positron Emission Tomography: Current Status of Research, Clinical Applications, and Future Perspectives. Semin. Nucl. Med. 2011, 41, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Domingo, R.J.; Reilly, R.M. Pre-targeted radioimmunotherapy of human colon cancer xenografts in athymic mice using streptavidin-CC49 monoclonal antibody and 90Y-DOTA-biotin. Nucl. Med. Commun. 2000, 21, 89–96. [Google Scholar] [CrossRef]
- Knör, S.; Modlinger, A.; Poethko, T.; Schottelius, M.; Wester, H.-J.; Kessler, H. Synthesis of Novel 1,4,7,10-Tetraazacyclodecane-1,4,7,10-Tetraacetic Acid (DOTA) Derivatives for Chemoselective Attachment to Unprotected Polyfunctionalized Compounds. Chem. Eur. J. 2007, 13, 6082–6090. [Google Scholar] [CrossRef]
- Lee, D.S.; Im, H.J.; Lee, Y.S. Radionanomedicine: Widened perspectives of molecular theragnosis. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 795–810. [Google Scholar] [CrossRef]
- Kleynhans, J.; Grobler, A.F.; Ebenhan, T.; Sathekge, M.M.; Zeevaart, J.R. Radiopharmaceutical enhancement by drug delivery systems: A review. J. Control. Release 2018, 287, 177–193. [Google Scholar] [CrossRef] [Green Version]
- McDevitt, M.R.; Sgouros, G.; Sofou, S. Targeted and nontargeted α-particle therapies. Annu. Rev. Biomed. Eng. 2018, 20, 73–93. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- ASTM. Standard Practice for Use of the Alanine-EPR Dosimetry System; ASTM International: West Conshohocken, PA, USA, 2012; ISO/ASTM51607-13. [Google Scholar]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Ulanski, P.; Bothe, E.; Hildenbrand, K.; Rosiak, J.M.; von Sonntag, C. Hydroxyl radical-induced reactions of poly(acrylic acid); A pulse radiolysis, EPR and product study. Part I. Deoxygenated aqueous solutions. J. Chem. Soc. Perkin Trans. 2 1996, 1, 13–22. [Google Scholar] [CrossRef]
- Arndt, K.-F.; Schmidt, T.; Reichelt, R. Thermo-sensitive poly(methyl vinyl ether) micro-gel formed by high energy radiation. Polymer (Guildf). 2001, 42, 6785–6791. [Google Scholar] [CrossRef]
- Dispenza, C.; Grimaldi, N.; Sabatino, M.-A.; Todaro, S.; Bulone, D.; Giacomazza, D.; Przybytniak, G.; Alessi, S.; Spadaro, G. Studies of network organization and dynamics of e-beam crosslinked PVPs: From macro to nano. Radiat. Phys. Chem. 2012, 81, 1349–1353. [Google Scholar] [CrossRef] [Green Version]
- Dispenza, C.; Sabatino, M.A.; Grimaldi, N.; Bulone, D.; Bondi, M.L.; Casaletto, M.P.; Rigogliuso, S.; Adamo, G.; Ghersi, G. Minimalism in radiation synthesis of biomedical functional nanogels. Biomacromolecules 2012, 13, 1805–1817. [Google Scholar] [CrossRef] [Green Version]
- Ghaffarlou, M.; Sütekin, S.D.; Güven, O. Preparation of nanogels by radiation-induced cross-linking of interpolymer complexes of poly(acrylic acid) with poly(vinyl pyrrolidone) in aqueous medium. Radiat. Phys. Chem. 2018, 142, 130–136. [Google Scholar] [CrossRef]
- An, J.-C.; Waver, A.; Kim, B.; Barkatt, A.; Poster, D.; Vreeland, W.N.; Silverman, J.; Al-Sheikhly, M. Radiation-induced synthesis of poly(vinylpyrrolidone) nanogels. Polymer 2011, 52, 5746–5755. [Google Scholar] [CrossRef]
- von Sonntag, C. The Chemical Basis of Radiation Biology; Taylor and Francis: London, UK, 1987. [Google Scholar]
- Ulanski, P.; Bothe, E.; Hildenbrand, K.; Rosiak, J.M.; von Sonntag, C. Hydroxyl-radical-induced reactions of poly(acrylic acid); A pulse radiolysis, EPR and product study. Part II. Oxygenated aqueous solutions. J. Chem. Soc. Perkin Trans. 2 1996, 1, 23–28. [Google Scholar] [CrossRef]
- Molyneux, P. Water-soluble synthetic polymers. In Properties and Applications; CRC Press. Inc.: Boca Raton, FL, USA, 1987. [Google Scholar]
- Oosawa, F. Polyelectrolytes; Marcel Dekker: New York, NY, USA, 1971. [Google Scholar]
- Burchard, W. Solution properties of branched macromolecules. Adv. Polym. Sci. 1999, 143, 114–194. [Google Scholar] [CrossRef]
- Moharram, M.A.; Balloomal, L.S.; El-Gendy, H.M. Infrared study of the complexation of poly(acrylic acid) with poly(acrylamide). J. Appl. Polym. Sci. 1996, 59, 987–990. [Google Scholar] [CrossRef]
- Dong, J.; Ozaki, Y.; Nakashima, K. Infrared, Raman, and near-infrared spectroscopic evidence for the coexistence of various hydrogen-bond forms in poly(acrylic acid). Macromolecules 1997, 30, 1111–1117. [Google Scholar] [CrossRef]
- Mojumdar, S.C.; Raki, L. Preparation, thermal, spectral and microscopic studies of calcium silicate hydrate–poly (acrylic acid) nanocomposite materials. J. Therm. Anal. Calorim. 2006, 85, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Refat, M.S.; Sadeek, S.A.; Khater, H.M. Electronic, infrared, and 1HNMR spectral studies of the novel charge-transfer complexes of o-tolidine and p-toluidine with alternation π-acceptors (3,5-dinitro benzoic acid and 2,6-dichloroquinone-4-chloroimide) in CHCl3 solvent. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 778–788. [Google Scholar] [CrossRef]
- Singh, N.; Khan, I.M.; Ahmad, A. Spectrophotometric and spectroscopic studies of charge transfer complexes of p-toluidine as an electron donor with picric acid as an electron acceptor in different solvents. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 1347–1353. [Google Scholar] [CrossRef]
- Zoromba, M.S. Novel and economic acid-base indicator based on (p-toluidine) oligomer: Synthesis; characterization and solvatochromism applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 187, 61–67. [Google Scholar] [CrossRef]
- Thomas, O.; Brogat, M. Chapter 12—UV Spectra Library. In UV-Visible Spectrophotometry of Water and Wastewater; Thomas, O., Burgess, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 379–517. ISBN 978-0-444-63897-7. [Google Scholar]
- Vandenbelt, J.M.; Henrich, C.; Vanden Berg, S.G. Comparison of pKa values determined by electrometric titration and ultraviolet absorption methods. Anal. Chem. 1954, 26, 726–727. [Google Scholar] [CrossRef]
- Liu, A.; Honma, I.; Ichihara, M.; Zhou, H. Poly(acrylic acid)-wrapped multi-walled carbon nanotubes composite solubilization in water: Definitive spectroscopic properties. Nanotechnology 2006, 17, 2845–2849. [Google Scholar] [CrossRef]
- Kavitha, T.; Kang, I.-K.; Park, S.-Y. Poly (acrylic acid)-grafted graphene oxide as an intracellular protein carrier. Langmuir 2014, 30, 402–409. [Google Scholar] [CrossRef]
- Filippov, A.; Munavirov, B.; Sparrman, T.; Ishmuhametova, V.; Rudakova, M.; Shriram, P.; Tavelin, S. Interaction of a poly(acrylic acid) oligomer with dimyristoylphosphatidylcholine bilayers. Langmuir 2011, 27, 3754–3761. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.J.; Mobli, M. Modelling 1H NMR Spectra of Organic Compounds: Theory, Applications and NMR Prediction Software; Wiley: Chichester, UK, 2008. [Google Scholar]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An automated force field topology builder (ATB) and repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Koziara, K.B.; Stroet, M.; Malde, A.K.; Mark, A.E. Testing and validation of the automated topology builder (ATB) version 2.0: Prediction of hydration free enthalpies. J. Comput. Aided Mol. Des. 2014, 28, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Canzar, S.; El-Kebir, M.; Pool, R.; Elbassioni, K.; Malde, A.K.; Mark, A.E.; Geerke, D.P.; Stougie, L.; Klau, G.W. Charge Group Partitioning in Biomolecular Simulation. J. Comput. Biol. 2013, 20, 188–198. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Nava, H.; Ferro-Flores, G.; Ramírez, F.D.M.; Ocampo-García, B.; Santos-Cuevas, C.; Aranda-Lara, L.; Azorín-Vega, E.; Morales-Avila, E.; Isaac-Olivé, K. 177Lu-dendrimer conjugated to folate and bombesin with gold nanoparticles in the dendritic cavity: A potential theranostic radiopharmaceutical. J. Nanomater. 2016, 2016, 1039258. [Google Scholar] [CrossRef] [Green Version]
- Aranda-Lara, L.; Ferro-Flores, G.; Azorín-Vega, E.; de Maria Ramírez, F.; Jiménez-Mancilla, N.; Ocampo-García, B.; Santos-Cuevas, C.; Isaac-Olivé, K. Synthesis and evaluation of Lys1(α,γ-Folate)Lys3(177Lu-DOTA)-Bombesin(1-14) as a potential theranostic radiopharmaceutical for breast cancer. Appl. Radiat. Isot. 2016, 107, 214–219. [Google Scholar] [CrossRef]
- Hajiramezanali, M.; Atyabi, F.; Mosayebnia, M.; Akhlaghi, M.; Geramifar, P.; Jalilian, A.R.; Mazidi, S.M.; Yousefnia, H.; Shahhosseini, S.; Beiki, D. (68)Ga-radiolabeled bombesin-conjugated to trimethyl chitosan-coated superparamagnetic nanoparticles for molecular imaging: Preparation, characterization and biological evaluation. Int. J. Nanomed. 2019, 14, 2591–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hixon, J.; Reshetnyak, Y.K. Algorithm for the analysis of tryptophan fluorescence spectra and their correlation with protein structural parameters. Algorithms 2009, 2, 1155–1176. [Google Scholar] [CrossRef] [Green Version]
- Accardo, A.; Mannucci, S.; Nicolato, E.; Vurro, F.; Diaferia, C.; Bontempi, P.; Marzola, P.; Morelli, G. Easy formulation of liposomal doxorubicin modified with a bombesin peptide analogue for selective targeting of GRP receptors overexpressed by cancer cells. Drug Deliv. Transl. Res. 2019, 9, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Carver, J.A. The conformation of bombesin in solution as determined by two-dimensional 1H-NMR techniques. Eur. J. Biochem. 1987, 168, 193–199. [Google Scholar] [CrossRef]
- Valliant, J.F.; Riddoch, R.W.; Hughes, D.W.; Roe, D.G.; Fauconnier, T.K.; Thornback, J.R. The solid phase synthesis and NMR spectroscopy of a 99Tc chelate–bombesin derived peptide conjugate. Inorg. Chim. Acta 2001, 325, 155–163. [Google Scholar] [CrossRef]
- Aime, S.; Botta, M.; Cravotto, G.; Frullano, L.; Giovenzana, G.B.; Crich, S.G.; Palmisano, G.; Sisti, M. Gadolinium(III) complexes of dota-derived N-sulfonylacetamides (H 4(dota-NHSO2R) = 10-{2-[(R)sulfonylamino]-2-oxoethyl}-1,4, 7,10-tetraazacyclododecane-1,4,7-triacetic acid): A new class of relaxation agents for magnetic resonance imaging applications. Helv. Chim. Acta 2005, 88, 588–603. [Google Scholar] [CrossRef]










| Sample | NG Carboxylate Groups | DMT/NMM/TsO− | p-Toluidine |
|---|---|---|---|
| NG/ca/T 1/1/1 | 1 | 1 | 1 |
| NG/ca/T 2/1/2 | 2 | 1 | 2 |
| NG/ca/T 10/1/10 | 10 | 1 | 10 |
| NG/ca/T 10/10/1 | 10 | 10 | 1 |
| Name of the Sample | Molar Ratio | Source and Type of Bombesin Derivative | |
|---|---|---|---|
| Carboxylate Groups of PAA Nanogel | DOTA-Bombesin Derivative | ||
| NGBD100H | 100 | 1 | in-house synthesized, with DOTA |
| NGB100H | 100 | 1 | in-house synthesized, without DOTA |
| NGBD100E | 100 | 1 | supplied by external company, with DOTA |
| NGBD500E | 500 | 1 | supplied by external company, with DOTA |
| NGBD1000E | 1000 | 1 | supplied by external company, with DOTA |
| Sample | Mw [Da = g · mol−1] | Rg [nm] | Rh [nm] | ρcoil [g · mol−1 nm−3] |
|---|---|---|---|---|
| Non-irradiated polymer | (8.87 ± 1.55) × 105 | 115 ± 17 | 42 ± 3 | 0.14 ± 0.04 |
| Nanogels irradiated at 5.3 kGy (NG_I) | (1.26 ± 0.09) × 106 | 94 ± 5 | 45 ± 4 | 0.36 ± 0.07 |
| Nanogels irradiated at 5.4 kGy | (1.13 ± 0.11) × 106 | 86 ± 22 | 35 ± 1 | 0.43 ± 0.31 |
| Nanogels irradiated at 5.4 kGy and re-filtered (NG_II) | (3.83 ± 0.34) × 105 | 41 ± 2 | 30 ± 4 | 1.35 ± 0.28 |
| Sample | Z-Average Rh (nm) |
|---|---|
| NG_I | 186 ± 19 |
| NG/ca/T 1/1/1 | 127 ± 18 |
| NG/ca/T 2/1/2 | 109 ± 7 |
| NG/ca/T 10/1/10 | 100 ± 9 |
| NG/ca/T 10/10/1 | 105 ± 3 |
| Sample | Z-Average Rh [nm] |
|---|---|
| NG_I | 186 ± 19 |
| NGBD100E | 212 ± 36 |
| NGBD500E | 176 ± 19 |
| NGBD1000E | 217 ± 25 |
| NG_II | 113 ± 43 |
| NGBD100H | 102 ± 9 |
| NGB100H | 145 ± 1 |
| Sample | Expected Bombesin Concentration at 100% Efficiency (mg/mL) | Estimated Bombesin Concentration from UV-Vis Data (mg/mL) | Approximate Coupling Efficiency (%) |
|---|---|---|---|
| NGBD100E | 0.281 | 0.252 | 90 |
| NGBD500E | 0.061 | 0.067 | 111 |
| NGBD1000E | 0.031 | 0.015 | 47 |
| NGBD100H | 0.313 | 0.203 | 65 |
| NGB100H | 0.202 | 0.105 | 52 |
| NGBD100E | NGBD500E | NGBD1000E | NG | |
|---|---|---|---|---|
| NGBD:Lu [mol:mol] | 13.9 | 3.7 | 0.8 | 0 |
| [177Lu]Lu-sample | 98.1 ± 0.7 | 57.4 ± 5.3 | 8.1 ± 0.6 | 0.7 ± 0.1 |
| [177Lu]Lu-BD | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| [177Lu]Lu free | 2.0 ± 0.6 | 42.6 ± 5.3 | 91.9 ± 0.6 | 99.3 ± 0.1 |
| Activity for Labeling (GBq mg−1) | |||||
|---|---|---|---|---|---|
| 19.64 | 9.31 | 0.78 | 0.68 | 0.18 | |
| BD:Lu [mol:mol] | 1.3 | 2.5 | 21.7 | 56.5 | 141 |
| [177Lu]Lu-NGBD100E [%] | 13.8 ± 1.2 | 20.2 ± 1.7 | 99.0 ± 1.1 | 98.1 ± 0.7 | 99.5 ± 0.7 |
| SA [GBq mg−1] | 2.71 | 1.88 | 0.77 | 0.67 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matusiak, M.; Rurarz, B.P.; Kadłubowski, S.; Wolszczak, M.; Karczmarczyk, U.; Maurin, M.; Kolesińska, B.; Ulański, P. Synthesis and Properties of Targeted Radioisotope Carriers Based on Poly(Acrylic Acid) Nanogels. Pharmaceutics 2021, 13, 1240. https://doi.org/10.3390/pharmaceutics13081240
Matusiak M, Rurarz BP, Kadłubowski S, Wolszczak M, Karczmarczyk U, Maurin M, Kolesińska B, Ulański P. Synthesis and Properties of Targeted Radioisotope Carriers Based on Poly(Acrylic Acid) Nanogels. Pharmaceutics. 2021; 13(8):1240. https://doi.org/10.3390/pharmaceutics13081240
Chicago/Turabian StyleMatusiak, Małgorzata, Beata P. Rurarz, Sławomir Kadłubowski, Marian Wolszczak, Urszula Karczmarczyk, Michał Maurin, Beata Kolesińska, and Piotr Ulański. 2021. "Synthesis and Properties of Targeted Radioisotope Carriers Based on Poly(Acrylic Acid) Nanogels" Pharmaceutics 13, no. 8: 1240. https://doi.org/10.3390/pharmaceutics13081240
APA StyleMatusiak, M., Rurarz, B. P., Kadłubowski, S., Wolszczak, M., Karczmarczyk, U., Maurin, M., Kolesińska, B., & Ulański, P. (2021). Synthesis and Properties of Targeted Radioisotope Carriers Based on Poly(Acrylic Acid) Nanogels. Pharmaceutics, 13(8), 1240. https://doi.org/10.3390/pharmaceutics13081240