A New Approach to Atopic Dermatitis Control with Low-Concentration Propolis-Loaded Cold Cream
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Composition of Beeswax
2.3. Chromatographic Profiling of Raw Beeswax and Quantitative Analysis of Artepillin C
2.4. Preparation of Standardized Green Propolis Extract
2.5. Anti-Inflammatory Activity of Green Propolis Extract
2.6. Preparation and Characterization of Cold Creams
2.6.1. Diameter of Droplets
2.6.2. Mechanical Properties
2.6.3. Bioadhesive Properties
2.7. Stability
2.8. Statistical Analysis
2.9. Preclinical Trial in AD Patients
2.9.1. Study Design
2.9.2. Study Population
2.9.3. Sample Size Calculation
2.9.4. Clinical Protocol
2.9.5. Instruments Used in the Clinical Trial
Sociodemographic and Clinical Data
Scoring Atopic Dermatitis (SCORAD)
Dermatology Life Quality Index (DLQI)
Evaluation of Skin Hydration
Evaluation of the General Morphology of Corneocytes
2.10. Statistical Analysis of the Data Obtained in the Clinical Study
3. Results
3.1. Composition of Beeswax
3.2. Chromatographic Profiling of Raw Beeswax and Quantitative Analysis of Artepillin C
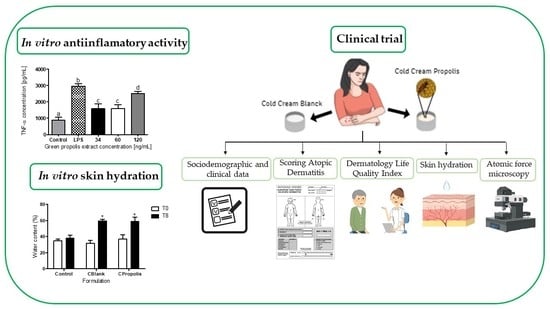
3.3. Anti-Inflammatory Activity of Green Propolis Extract
3.4. Characterization of the Cold Creams
3.5. Stability
3.6. Preclinical Trial in Humans
3.6.1. Characteristics of the Study Population
3.6.2. Evaluation of SCORAD, DLQI, and Skin Hydration
3.6.3. Assessment of General Corneocyte Morphology
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flohr, C.; Mann, J. New insights into the epidemiology of childhood atopic dermatitis. Allergy 2014, 69, 3–16. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Rajka, G. Diagnostic features of Atopic Dermatitis. Acta Derm. Venereol. Suppl. 1980, 60, 44–47. [Google Scholar] [CrossRef]
- Antunes, A.A.; Solé, D.; Carvalho, V.O.; Bau, A.E.K.; Kuschnir, F.C.; Mallozi, M.C.; Markus, J.R.; Silva, M.G.N.E.; Pires, M.C.; Mello, M.E.E.A.; et al. Updated practical guide on atopic dermatitis-Part I: Etiopathogenesis, clinical features, and diagnosis. Joint position paper of the Brazilian Association of Allergy and Immunology and the Brazilian Society of Pediatrics. Arq. Asmas Alerg. E Imunol. 2017, 1, 131–156. [Google Scholar] [CrossRef]
- Castro, A.P.M.; Solé, D.; Filho, N.A.R.; Jacob, C.M.A.; Rizzo, M.C.F.V.; Fernandes, M.D.F.M.; Vale, S.O.R. Practical guide for management of atopic dermatitis-conjunct opinion of allergologists from the Associação Brasileira de Alergia e Imunopatologia and Sociedade Brasileira de Pediatria. Rev. Bras. Alerg. Imunopatol. 2006, 29, 268–282. [Google Scholar]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Powers, C.E.; McShane, D.B.; Gilligan, P.H.; Burkhart, C.N.; Morrell, D.S. Microbiome and pediatric atopic dermatitis. J. Dermatol. 2015, 42, 1137–1142. [Google Scholar] [CrossRef] [Green Version]
- Proksch, E.; Jensen, J.M.; Elias, P.M. Skin lipids and epidermal differentiation in atopic dermatitis. Clin. Dermatol. 2003, 21, 134–144. [Google Scholar] [CrossRef]
- Batista, D.I.S.; Perez, L.; Orfali, R.L.; Zaniboni, M.C.; Samorano, L.P.; Pereira, N.V.; Sotto, M.N.; Ishizaki, A.S.; Oliveira, L.M.S.; Sato, M.N.; et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1091–1095. [Google Scholar] [CrossRef]
- Toda, M.; Leung, D.Y.M.; Molet, S.; Boguniewicz, M.; Taha, R.; Christodoulopoulos, P.; Fukuda, T.; Elias, J.A.; Hamid, Q.A. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J. Allergy Clin. Immunol. 2003, 111, 875–881. [Google Scholar] [CrossRef]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y.M. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.B.; Nadkarni, N.J.; Patil, S.P.; Godse, K.V.; Gautam, M.; Agarwal, S. Topical corticosteroids in dermatology. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 371–378. [Google Scholar] [CrossRef]
- Odedra, K.M. Current clinical practice in atopic dermatitis. Nurs. Stand. 2014, 28, 45–51. [Google Scholar] [CrossRef]
- Harper, J.I.; Ahmed, I.; Barclay, G.; Lacour, M.; Hoeger, P.; Cork, M.J.; Finlay, A.Y.; Wilson, N.J.E.; Graham-Brown, R.A.C.; Sowden, J.M.; et al. Cyclosporin for severe childhood atopic dermatitis: Short course versus continuous therapy. Br. J. Dermatol. 2000, 142, 52–58. [Google Scholar] [CrossRef]
- Moncrieff, G.; Cork, M.; Lawton, S.; Kokiet, S.; Daly, C.; Clark, C. Use of emollients in dry-skin conditions: Consensus statement. Clin. Exp. Dermatol. 2013, 38, 231–238. [Google Scholar] [CrossRef]
- Beheshti, A.; Shafigh, Y.; Abdollah Zangivand, A.; Samiee-Rad, F.; Hassanzadeh, G.; Shafigh, N. Comparison of topical sucralfate and silver sulfadiazine cream in second degree burns in rats. Adv. Clin. Exp. Med. 2013, 22, 481–487. [Google Scholar]
- Avila-Romay, A.; Alvarez-Franco, M.; Ruiz-Maldonado, R. Therapeutic Efficacy, Secondary Effects, and Patient Acceptability of 10% Sulfur in Either Pork Fat or Cold Cream for the Treatment of Scabies. Pediatr. Dermatol. 1991, 8, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, A.H.; Ghaninejad, H.; Kiani, A.; Daneshpazhooh, M.; Hosseini, S.H.; Noormohammadpoor, P. Comparison of topical 8-methoxypsoralen and narrowband ultraviolet B with narrowband ultraviolet B alone in treatment-resistant sites in plaque-type psoriasis: A placebo-controlled study. Photodermatol. Photoimmunol. Photomed. 2011, 27, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Koppes, S.A.; Charles, F.; Lammers, L.A.; Frings-Dresen, M.; Kezic, S.; Rustemeyer, T. Efficacy of a cream containing ceramides and magnesium in the treatment of mild to moderate atopic dermatitis: A randomized, double-blind, emollient- and hydrocortisone-controlled trial. Acta Derm. Venereol. 2016, 96, 948–953. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, K.; Tagami, H.; Agawa, Y.; Akazaki, S.; Ando, M.; Hayashi, A.; Hayashi, T.; Hirao, T.; Ishida, T.; Ito, K.; et al. Noninvasive biophysical assessments of the efficacy of a moisturizing cosmetic cream base for patients with atopic dermatitis during different seasons. Br. J. Dermatol. 2008, 158, 969–978. [Google Scholar] [CrossRef]
- Daleprane, J.B.; da Silva Freitas, V.; Pacheco, A.; Rudnicki, M.; Faine, L.A.; Dörr, F.A.; Ikegaki, M.; Salazar, L.A.; Ong, T.P.; Abdalla, D.S.P. Anti-atherogenic and anti-angiogenic activities of polyphenols from propolis. J. Nutr. Biochem. 2012, 23, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.S.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- de Barros, M.P.; Sousa, J.P.B.; Bastos, J.K.; de Andrade, S.F. Effect of Brazilian green propolis on experimental gastric ulcers in rats. J. Ethnopharmacol. 2007, 110, 567–571. [Google Scholar] [CrossRef]
- Orsatti, C.L.; Missima, F.; Pagliarone, A.C.; Bachiega, T.F.; Búfalo, M.C.; Araújo, J.P.; Sforcin, J.M. Propolis immunomodulatory action in vivo on toll-like receptors 2 and 4 expression and on pro-inflammatory cytokines production in mice. Phyther. Res. 2010, 24, 1141–1146. [Google Scholar] [CrossRef]
- Song, Y.S.; Park, E.H.; Hur, G.M.; Ryu, Y.S.; Kim, Y.M.; Jin, C. Ethanol extract of propolis inhibits nitric oxide synthase gene expression and enzyme activity. J. Ethnopharmacol. 2002, 80, 155–161. [Google Scholar] [CrossRef]
- Fitzpatrick, L.R.; Wang, J.; Le, T. Caffeic Acid Phenethyl Ester, an Inhibitor of Nuclear Factor-κB, Attenuates Bacterial Peptidoglycan Polysaccharide-Induced Colitis in Rats. J. Pharmacol. Exp. Ther. 2001, 299, 915–920. [Google Scholar]
- Duthie, G.G.; Gardner, P.T.; Kyle, J.A.M. Plant polyphenols: Are they the new magic bullet? Proc. Nutr. Soc. 2003, 62, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Hori, J.I.; Zamboni, D.S.; Carrão, D.B.; Goldman, G.H.; Berretta, A.A. The inhibition of inflammasome by Brazilian propolis (EPP-AF). Evid.-Based Complement. Altern. Med. 2013, 2013, 418508. [Google Scholar] [CrossRef] [Green Version]
- Garnier, N.; Cren-Olivé, C.; Rolando, C.; Regert, M. Characterization of archaeological beeswax by electron ionization and electrospray ionization mass spectrometry. Anal. Chem. 2002, 74, 4868–4877. [Google Scholar] [CrossRef]
- de Sousa, J.P.B.; Bueno, P.C.P.; Gregório, L.E.; da Silva Filho, A.A.; Furtado, N.A.J.C.; de Sousa, M.L.; Bastos, J.K. A reliable quantitative method for the analysis of phenolic compounds in Brazilian propolis by reverse phase high performance liquid chromatography. J. Sep. Sci. 2007, 30, 2656–2665. [Google Scholar] [CrossRef]
- Costa, P.; Almeida, M.O.; Lemos, M.; Arruda, C.; Casoti, R.; Somensi, L.B.; Boeing, T.; Mariott, M.; Stein, B.D.P.; de Souza, P.; et al. Artepillin C, drupanin, aromadendrin-4′-O-methyl-ether and kaempferide from Brazilian green propolis promote gastroprotective action by diversified mode of action. J. Ethnopharmacol. 2018, 226, 82–89. [Google Scholar] [CrossRef]
- OECD. Test No. 428: Skin Absorption: In Vitro Method; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2004; pp. 1–8. [Google Scholar] [CrossRef]
- Lemos, C.N.; Cubayachi, C.; Dias, K.; Mendonça, J.N.; Lopes, N.P.; Furtado, N.A.J.C.; Lopez, R.F.V. Iontophoresis-stimulated silk fibroin films as a peptide delivery system for wound healing. Eur. J. Pharm. Biopharm. 2018, 128, 147–155. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Amaral, M.H.; Sousa Lobo, J.M. Comparison between sensory and instrumental characterization of topical formulations: Impact of thickening agents. Int. J. Cosmet. Sci. 2016, 38, 389–398. [Google Scholar] [CrossRef]
- Heck, R.; Hermann, S.; Lunter, D.J.; Daniels, R. Film-forming formulations containing porous silica for the sustained delivery of actives to the skin. Eur. J. Pharm. Biopharm. 2016, 108, 1–8. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; The, C. group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- You, C.E.; Moon, S.H.; Lee, K.H.; Kim, K.H.; Park, C.W.; Seo, S.J.; Cho, S.H. Effects of emollient containing bee venom on atopic dermatitis: A double-blinded, randomized, base-controlled, multicenter study of 136 patients. Ann. Dermatol. 2016, 28, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Grimalt, R.; Mengeaud, V.; Cambazard, F. The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: A randomized controlled study. Dermatology 2006, 214, 61–67. [Google Scholar] [CrossRef]
- Szczepanowska, J.; Reich, A.; Szepietowski, J.C. Emollients improve treatment results with topical corticosteroids in childhood atopic dermatitis: A randomized comparative study. Pediatr. Allergy Immunol. 2008, 19, 614–618. [Google Scholar] [CrossRef]
- Wollenberg, A.; Oranje, A.; Deleuran, M.; Simon, D.; Szalai, Z.; Kunz, B.; Svensson, A.; Barbarot, S.; Von Kobyletzki, L.; Taieb, A.; et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 729–747. [Google Scholar] [CrossRef] [Green Version]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef]
- Franz, J.; Beutel, M.; Gevers, K.; Kramer, A.; Thyssen, J.P.; Kezic, S.; Riethmüller, C. Nanoscale alterations of corneocytes indicate skin disease. Ski. Res. Technol. 2016, 22, 174–180. [Google Scholar] [CrossRef]
- Koppes, S.A.; Ljubojević Hadžavdić, S.; Jakasa, I.; Franceschi, N.; Riethmüller, C.; Jurakić Tončic, R.; Marinovic, B.; Raj, N.; Rawlings, A.V.; Voegeli, R.; et al. Effect of allergens and irritants on levels of natural moisturizing factor and corneocyte morphology. Contact Dermat. 2017, 76, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Kucharska, A.Z.; Sokó, A.; Aw, B.; Mertas, A.; Czuba, Z.P.; Król, W. Chemical Composition and Anti-Inflammatory Effect of Ethanolic Extract of Brazilian Green Propolis on Activated J774A.1 Macrophages. Evid.-Based Complement. Altern. Med. 2013, 2013, 976415. [Google Scholar] [CrossRef]
- Berretta, A.A.; Arruda, C.; Miguel, F.G.; Baptista, N.; Nascimento, A.P.; Marquele-Oliveira, F.; Hori, J.I.; Barud, H.D.S.; Damaso, B.; Ramos, C.; et al. Functional Properties of Brazilian Propolis: From Chemical Composition Until the Market. In Superfood and Functional Food-An Overview of Their Processing and Utilization; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, F.C.; Bruschi, M.L.; Evangelista, R.C.; Gremião, M.P.D. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci. 2010, 46, 89. [Google Scholar] [CrossRef] [Green Version]
- Baroli, B. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J. Pharm. Sci. 2010, 99, 21–50. [Google Scholar] [CrossRef]
- Berdyshev, E.; Goleva, E.; Bronova, I.; Dyjack, N.; Rios, C.; Jung, J.; Taylor, P.; Jeong, M.; Hall, C.F.; Richers, B.N.; et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight 2018, 3, e98006. [Google Scholar] [CrossRef] [Green Version]
- Machado, J.L.; Assunção, A.K.M.; Da Silva, M.C.P.; Reis, A.S.D.; Costa, G.C.; Arruda, D.D.S.; Rocha, B.A.; Vaz, M.M.D.O.L.L.; Paes, A.M.D.A.; Guerra, R.N.M.; et al. Brazilian green propolis: Anti-inflammatory property by an immunomodulatory activity. Evid.-Based Complement. Altern. Med. 2012, 2012, 157652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Yang, B.; Wang, D.; Zhu, Y.; Miao, X.; Yang, W. The chemical composition of brazilian green propolis and its protective effects on mouse aortic endothelial cells against inflammatory injury. Molecules 2020, 25, 4612. [Google Scholar] [CrossRef]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; Kirschner, D.E.; et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Danso, M.O.; Van Drongelen, V.; Mulder, A.; Van Esch, J.; Scott, H.; Van Smeden, J.; El Ghalbzouri, A.; Bouwstra, J.A. TNF-α and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J. Investig. Dermatol. 2014, 134, 1941–1950. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Lei, Y.; Wu, Y.; Liang, H.; Li, J.; Pei, Y.; Li, Y.; Li, B.; Luo, X.; Liu, S. Beeswax: A potential self-emulsifying agent for the construction of thermal-sensitive food W/O emulsion. Food Chem. 2021, 349, 129203. [Google Scholar] [CrossRef]
- Hurler, J.; Škalko-Basnet, N. Potentials of Chitosan-Based Delivery Systems in Wound Therapy: Bioadhesion Study. J. Funct. Biomater. 2012, 3, 37–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dano, M.E.L.; dos Santos, R.S.; da Silva, J.B.; Junqueira, M.V.; de Souza Ferreira, S.B.; Bruschi, M.L. Design of emulgel platforms for local propolis delivery: The influence of type and concentration of carbomer. J. Mol. Liq. 2021, 334, 116025. [Google Scholar] [CrossRef]
- Gonçalves, G.M.S.; Srebernich, S.M.; Souza, J.A.D.M. Stability and sensory assessment of emulsions containing propolis extract and/or tocopheryl acetate. Braz. J. Pharm. Sci. 2011, 47, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Marquele-Oliveira, F.; Fonseca, Y.M.; De Freitas, O.; Fonseca, M.J.V. Development of topical functionalized formulations added with propolis extract: Stability, cutaneous absorption and in vivo studies. Int. J. Pharm. 2007, 342, 40–48. [Google Scholar] [CrossRef] [PubMed]
- de Groot, A.C. Propolis: A review of properties, applications, chemical composition, contact allergy, and other adverse effects. Dermatitis 2013, 24, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Nakamura, M.; Makino, T.; Hino, T.; Kagoura, M.; Morohashi, M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br. J. Dermatol. 2002, 147, 71–79. [Google Scholar] [CrossRef]







| Mechanical Properties | CBlank | CPropolis |
|---|---|---|
| Hardness (×10−1 N) | 4.9 ± 0.1 | 5.2 ± 0.1 |
| Compressibility (×10−1 N.mm) | 9.0 ± 0.3 | 9.5 ± 0.5 |
| Adhesiveness (×10−1 N.mm) | 5.4 ± 0.2 | 5.7 ± 0.2 |
| Cohesiveness | 6.5 ± 0.1 | 6.6 ± 0.1 |
| Population | Control Group (n = 8) | Frequency (%) | Intervention Group (n = 8) | Frequency (%) | p-Value |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 5 | 62.5 | 6 | 75 | 0.999 * |
| Male | 3 | 37.5 | 2 | 25 | |
| Race | |||||
| White | 7 | 87.5 | 6 | 75 | 0.999 * |
| Black | 1 | 12.5 | 2 | 25 | |
| Marital Status | |||||
| Married | 4 | 50 | 3 | 37.5 | 0.120 |
| Single | 2 | 25 | 5 | 62.5 | |
| Divorced | 2 | 25 | 0 | 0 | |
| Education Level | |||||
| Incomplete Elementary School | 1 | 12.5 | 1 | 12.5 | 1.000 |
| Elementary School | 1 | 12.5 | 0 | 0 | |
| Middle School | 5 | 62.5 | 6 | 75 | |
| Higher Education | 1 | 12.5 | 1 | 12.5 | |
| Frequency of visits to the dermatologist (Times/Year) | |||||
| 0–4 | 6 | 75 | 4 | 50 | 0.999 |
| 5–7 | 1 | 12.5 | 1 | 12.5 | |
| 8–12 | 1 | 12.5 | 3 | 37.5 |
| Patient | Control Group | Intervention Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Outset | 60 Days | Outset | 60 Days | |||||
| Points | Classification | Points | Classification | Points | Classification | Points | Classification | |
| 1 | 42.0 | Moderate | 52.5 | Severe | 65.0 | Severe | 42.0 | Moderate |
| 2 | 47.2 | Moderate | 32.5 | Moderate | 62.5 | Severe | 58.5 | Severe |
| 3 | 45.5 | Moderate | 49.0 | Moderate | 12.4 | Mild | 12.9 | Mild |
| 4 | 13.0 | Mild | 25.1 | Moderate | 29.0 | Moderate | 32.5 | Moderate |
| 5 | - | - | - | - | 21.5 | Mild | 19.0 | Mild |
| Mean ± SD | 37 ± 16 | 40 ± 13 | 38 ± 24 | 33 ± 18 | ||||
| Patient | Control Group | Intervention Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Outset | 60 Days | Outset | 60 Days | |||||
| Points | Effect on DLQI | Points | Effect on DLQI | Points | Effect on DLQI | Points | Effect on DLQI | |
| 1 | 2 | Small | 1 | No effect | 15 | Very large | 1 | No effect |
| 2 | 8 | Moderate | 6 | Moderate | 17 | Very large | 16 | Very large |
| 3 | 15 | Very large | 7 | Moderate | 8 | Moderate | 3 | Small |
| 4 | 15 | Very large | 1 | No effect | 10 | Moderate | 4 | Small |
| 5 | - | - | - | - | 5 | Small | 0 | No effect |
| Mean ± SD | 10 ± 6 | 4 ± 3 | 11 ± 5 | 5 ± 6 | ||||
| Patient | Control Group | Intervention Group | ||
|---|---|---|---|---|
| Outset | 60 Days | Outset | 60 Days | |
| 1 | 28 | 14 | 18 | 27 |
| 2 | 39 | 31 | 46 | 48 |
| 3 | 58 | 56 | 86 | 36 |
| 4 | 44 | 27 | 16 | 30 |
| 5 | - | - | 51 | 45 |
| Mean ± SD | 42 ± 12 | 32 ± 17 | 43 ± 29 | 37 ± 9 |
| Variable | Patient | |||
|---|---|---|---|---|
| Patient 1 | Patient 2 | |||
| Outset | After 60 Days | Outset | After 60 Days | |
| SCORAD | 42 (moderate) | 52.5 (severe) | 47.2 (moderate) | 32.5 (moderate) |
| DLQI | 2 (small) | 1 (no effect) | 8 (moderate) | 6 (moderate) |
| Hydration | 28 | 14 | 39 | 31 |
| Variable | Patient | |||
| Patient 3 | Patient 4 | |||
| Outset | After 60 Days | Outset | After 60 Days | |
| SCORAD | 45.5 (moderate) | 49 (moderate) | 13 (mild) | 25.1 (moderate) |
| DLQI | 15 (very large) | 7 (moderate) | 15 (very large) | 1 (no effect) |
| Hydration | 58 | 56 | 44 | 27 |
| Variable | Patient | |||
|---|---|---|---|---|
| Patient 1 | Patient 2 | |||
| Outset | After 60 Days | Outset | After 60 Days | |
| SCORAD | 65 (severe) | 42 (moderate) | 62.5 (severe) | 58.5 (severe) |
| DLQI | 15 (very large) | 1 (no effect) | 17 (very large) | 16 (very large) |
| Hydration | 18 | 27 | 46 | 48 |
| Variable | Patient 3 | Patient 4 | ||
| Outset | After 60 Days | Outset | After 60 Days | |
| SCORAD | 12.4 (mild) | 12.9 (mild) | 29 (moderate) | 32.5 (moderate) |
| DLQI | 8 (moderate) | 3 (small) | 10 (moderate) | 4 (small) |
| Hydration | 86 | 36 | 16 | 30 |
| Variable | Patient 5 | |||
| Outset | Outset | |||
| SCORAD | 21.5 (mild) | 21.5 (mild) | ||
| DLQI | 5 (small) | 5 (small) | ||
| Hydration | 51 | 51 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, B.A.; Lemos, C.N.; Dalmolin, L.F.; Arruda, C.; Brait, Í.S.C.; Cazarim, M.d.S.; da Cruz-Cazarim, E.L.C.; Bueno, P.C.P.; Júnior, M.P.; Pereira, L.R.L.; et al. A New Approach to Atopic Dermatitis Control with Low-Concentration Propolis-Loaded Cold Cream. Pharmaceutics 2021, 13, 1346. https://doi.org/10.3390/pharmaceutics13091346
Martin BA, Lemos CN, Dalmolin LF, Arruda C, Brait ÍSC, Cazarim MdS, da Cruz-Cazarim ELC, Bueno PCP, Júnior MP, Pereira LRL, et al. A New Approach to Atopic Dermatitis Control with Low-Concentration Propolis-Loaded Cold Cream. Pharmaceutics. 2021; 13(9):1346. https://doi.org/10.3390/pharmaceutics13091346
Chicago/Turabian StyleMartin, Bianca Aparecida, Camila Nunes Lemos, Luciana Facco Dalmolin, Caroline Arruda, Íris Sperchi Camilo Brait, Maurílio de Souza Cazarim, Estael Luzia Coelho da Cruz-Cazarim, Paula Carolina Pires Bueno, Maurílio Polizello Júnior, Leonardo Régis Leira Pereira, and et al. 2021. "A New Approach to Atopic Dermatitis Control with Low-Concentration Propolis-Loaded Cold Cream" Pharmaceutics 13, no. 9: 1346. https://doi.org/10.3390/pharmaceutics13091346
APA StyleMartin, B. A., Lemos, C. N., Dalmolin, L. F., Arruda, C., Brait, Í. S. C., Cazarim, M. d. S., da Cruz-Cazarim, E. L. C., Bueno, P. C. P., Júnior, M. P., Pereira, L. R. L., Cardili, R. N., & Fonseca Vianna Lopez, R. (2021). A New Approach to Atopic Dermatitis Control with Low-Concentration Propolis-Loaded Cold Cream. Pharmaceutics, 13(9), 1346. https://doi.org/10.3390/pharmaceutics13091346