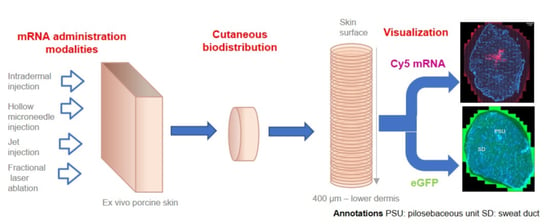
Effect of mRNA Delivery Modality and Formulation on Cutaneous mRNA Distribution and Downstream eGFP Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procuring and Processing Viable Porcine Skin
2.3. Experimental Conditions
2.3.1. mRNA
2.3.2. mRNA Delivery Techniques
- Intradermal injections. Intradermal (ID) injection and hollow microneedle intradermal injection systems (MicronJet™600) were selected among “invasive” techniques. A 26 G × 3/8″ needle was used to inject mRNA formulations into the dermal region of porcine skin. Although ID injection is a commonly used technique for the targeted delivery of actives into the dermis (e.g., BCG and other vaccines; Mendel–Mantoux tuberculin test; local anesthesia and aesthetic surgery) [36], it requires specific training – for example, the injection angle must be low (10–15°) and the injection volumes should be < 0.5 mL. Another ID injection-based delivery system tested was the FDA- and EMA-approved MicronJet™600 (NanoPass Technologies, Ltd., Nes Ziona, Israel). This consists of an array of three 600 μm microneedles, made from silicon, that can be easily mounted on a syringe. The microneedles are 600 μm in length and already beveled to facilitate insertion into the skin at the correct angle [37]. This system has already been proven effective for mRNA delivery [38].
- Jet injector. A needle-free jet injector system, Dermojet® developed by AKRA DERMOJET (Pau, France), was also used to deliver mRNA formulations. Liquid-jet injectors use compressed gas or spring to generate a high-velocity jet (with velocities ranging from 100 to 200 m/s) propelled from a nozzle with a pressure of about 1420 psi. Depending on the jet velocity and orifice diameter, the jet can be delivered into the dermis or deeper [39]. Successful drug delivery and good clinical outcomes have already been achieved using this device [40]. mRNA delivery was investigated using 2 different “heads”: one with a single nozzle and the other with a triple nozzle, i.e., enabling simultaneous injection at three different sites.
- Fractional laser ablation. Minimally invasive fractional laser ablation has also been demonstrated as being able to deliver macromolecules [41,42,43,44] and positive clinical outcomes have been reported for the laser assisted delivery of etanercept for the treatment of psoriasis in a phase 1 study [45]. Low-intensity Erbium:YAG (solid-state erbium-doped yttrium aluminum garnet) lasers emitting light at 2940 nm are routinely used for skin ablation. Each pulse can ablate a reproducible amount of tissue; thus, the pore depth can be controlled [39,46]. Using this technology, Pantec Biosolutions AG (Ruggell, Liechtenstein) developed the P.L.E.A.S.E® system (Precise Laser Epidermal System) for delivery of low and high molecular weight molecules. The device can create an array of 150 μm diameter micropores on a small skin area. The pore depth is controlled by modulating (i) the number of pulses per pore and (ii) pulse energy or fluence (J/cm2). The latter depends on the pulse duration (μs) and repetition rate (Hz). To obtain minimally invasive painless ablation, the pore depth must be limited so as not to reach the sensitive nerve endings situated in the dermis. Finally, the number of pores created per unit area determines the fraction of skin surface that is removed, and this is defined as the fractional ablated area (%). Thus, selectively ablating superficial layers of skin would hypothetically provide direct access to epidermal cells able to express the target protein.
2.4. Sample Processing
2.4.1. Snap Freezing and Cryosectioning
2.4.2. Tissue Characterization: Hematoxylin-Eosin Staining
2.4.3. Cy5 Labeled mRNA Delivery: Staining
2.4.4. eGFP Expression: Immunochemical Staining
2.4.5. Widefield Microscopy and Confocal Laser Scanning Microscopy
2.4.6. Data Processing
3. Results and Discussion
3.1. Tissue Characterization
3.2. Effect of Different Delivery Techniques on Skin Distribution of Cy5 Labeled mRNA and eGFP Expression
3.2.1. Intradermal Injections
3.2.2. Jet Injection
3.2.3. Fractional Laser Ablation
3.3. Semi-Quantification of Cy5 Labeled mRNA Delivery and Subsequent eGFP Expression
3.4. Clinical/Preclinical Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AK | Actinic keratosis |
| ASO | Antisense oligonucleotide |
| BCC | Basal cell carcinoma |
| CBM | Cutaneous biodistribution method |
| CLSM | Confocal laser scanning microscopy |
| Cy5 | Cyanine 5 |
| DAPI | 4′,6-diamidino-2-phénylindole |
| DEJ | Dermo-epithelial junction |
| DNA | Deoxyribonucleic acid |
| eGFP | Enhanced green fluorescent protein |
| EMA | European medical agency |
| Er: YAG | Solid state erbium-doped yttrium aluminium garnet |
| FDA | Food and Drug Administration |
| FM | Fluorescent microscopy |
| H&E | Hematoxylin and eosin |
| HPLC | High-pressure liquid chromatography |
| ID | Intradermal |
| INF-α | Interferon-α |
| IVT | In vitro transcribed |
| mIgG | Mouse immunoglobulin G |
| mRNA | Messenger ribonucleic acid |
| NGS | Normal goat serum |
| PBS | Phosphate buffer saline |
| PBST | Phosphate buffer saline with Tween 20 |
| PD | Papillary dermis |
| PSU | Pilosebaceous unit |
| RD | Reticular dermis |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SCC | Squamous cell carcinoma |
| SC | Stratum corneum |
| SD | Sweat duct |
| siRNA | Small interfering ribonucleic acid |
| VE | Viable epidermis |
| VEGF-A | Vascular endothelial growth factor-A |
References
- Smith, C.H.; Yiu, Z.Z.N.; Bale, T.; Burden, A.D.; Coates, L.C.; Edwards, W.; MacMahon, E.; Mahil, S.K.; McGuire, A.; Murphy, R.; et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2020: A rapid update. Br. J. Dermatol. 2020, 183, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guttman-Yassky, E. Efficacy of biologics in atopic dermatitis. Expert Opin. Biol. Ther. 2020, 20, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.E.; Fernández-Peñas, P. Adverse Reactions to Biologics: Melanoma (Ipilimumab, Nivolumab, Pembrolizumab). Curr. Probl. Dermatol. 2018, 53, 82–92. [Google Scholar] [CrossRef]
- Kamata, M.; Tada, Y. Safety of biologics in psoriasis. J. Dermatol. 2018, 45, 279–286. [Google Scholar] [CrossRef]
- Bachhav, Y.G.; Heinrich, A.; Kalia, Y.N. Controlled intra- and transdermal protein delivery using a minimally invasive Erbium: YAG fractional laser ablation technology. Eur. J. Pharm. Biopharm. 2013, 84, 355–364. [Google Scholar] [CrossRef]
- Song, Y.; Hemmady, K.; Puri, A.; Banga, A.K. Transdermal delivery of human growth hormone via laser-generated micropores. Drug Deliv. Transl. Res. 2018, 8, 450–460. [Google Scholar] [CrossRef]
- Lapteva, M.; Sallam, M.A.; Goyon, A.; Guillarme, D.; Veuthey, J.L.; Kalia, Y.N. Non-invasive targeted iontophoretic delivery of cetuximab to skin. Expert Opin. Drug Deliv. 2020, 17, 589–602. [Google Scholar] [CrossRef]
- Cázares-Delgadillo, J.; Naik, A.; Ganem-Rondero, A.; Quintanar-Guerrero, D.; Kalia, Y.N. Transdermal delivery of cytochrome C–A 12.4 kDa protein—Across intact skin by constant-current iontophoresis. Pharm. Res. 2007, 24, 1360–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, S.; Kalia, Y.N. Non-invasive iontophoretic delivery of enzymatically active ribonuclease A (13.6 kDa) across intact porcine and human skins. J. Control. Release 2010, 145, 203–209. [Google Scholar] [CrossRef]
- FDA. What is Gene Therapy? Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/what-gene-therapy (accessed on 3 November 2021).
- EMA. Glybera. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/glybera#assessment-history-section (accessed on 3 November 2021).
- EMA. Strimvelis. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/strimvelis (accessed on 3 November 2021).
- EMA. Luxturna. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/luxturna (accessed on 3 November 2021).
- EMA. Zolgensma. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zolgensma (accessed on 3 November 2021).
- Patil, S.D.; Rhodes, D.G.; Burgess, D.J. DNA-based therapeutics and DNA delivery systems: A comprehensive review. AAPS J. 2005, 7, E61–E77. [Google Scholar] [CrossRef] [Green Version]
- Hackett, P.B.; Largaespada, D.A.; Switzer, K.C.; Cooper, L.J. Evaluating risks of insertional mutagenesis by DNA transposons in gene therapy. Transl. Res. 2013, 161, 265–283. [Google Scholar] [CrossRef] [Green Version]
- Tavernier, G.; Wolfrum, K.; Demeester, J.; De Smedt, S.C.; Adjaye, J.; Rejman, J. Activation of pluripotency-associated genes in mouse embryonic fibroblasts by non-viral transfection with in vitro-derived mRNAs encoding Oct4, Sox2, Klf4 and cMyc. Biomaterials 2012, 33, 412–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Guan, S.; Rosenecker, J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017, 24, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P.H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982, 1, 841–845. [Google Scholar] [CrossRef]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Desigaux, L.; Sainlos, M.; Lambert, O.; Chevre, R.; Letrou-Bonneval, E.; Vigneron, J.P.; Lehn, P.; Lehn, J.M.; Pitard, B. Self-assembled lamellar complexes of siRNA with lipidic aminoglycoside derivatives promote efficient siRNA delivery and interference. Proc. Natl. Acad. Sci. USA 2007, 104, 16534–16539. [Google Scholar] [CrossRef] [Green Version]
- Leus, N.G.; Morselt, H.W.; Zwiers, P.J.; Kowalski, P.S.; Ruiters, M.H.; Molema, G.; Kamps, J.A. VCAM-1 specific PEGylated SAINT-based lipoplexes deliver siRNA to activated endothelium in vivo but do not attenuate target gene expression. Int. J. Pharm. 2014, 469, 121–131. [Google Scholar] [CrossRef]
- EMA. Onpattro. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/onpattro (accessed on 3 November 2021).
- EMA. Tegsedi. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tegsedi (accessed on 3 November 2021).
- EMA. Spinraza. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/spinraza (accessed on 3 November 2021).
- Yu, A.M.; Jian, C.; Yu, A.H.; Tu, M.J. RNA therapy: Are we using the right molecules? Pharmacol. Ther. 2019, 196, 91–104. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial Delivery Systems for mRNA Vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- EMA. COVID-19 Vaccines: Authorised. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised (accessed on 3 November 2021).
- ClinicalTrials.gov. An Efficacy Study of Adjuvant Treatment with the Personalized Cancer Vaccine mRNA-4157 and Pembrolizumab in Participants with High-Risk Melanoma. Available online: https://clinicaltrials.gov/ct2/show/NCT03897881 (accessed on 3 November 2021).
- Moderna. Moderna’s Pipeline. Available online: https://www.modernatx.com/pipeline (accessed on 3 November 2021).
- Gan, L.-M.; Lagerström-Fermér, M.; Carlsson, L.G.; Arfvidsson, C.; Egnell, A.-C.; Rudvik, A.; Kjaer, M.; Collén, A.; Thompson, J.D.; Joyal, J.; et al. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat. Commun. 2019, 10, 871. [Google Scholar] [CrossRef]
- Hochmann, S.; Mittermeir, M.; Santic, R.; Koszik, F.; Griessner, L.; Sonderegger, A.S.; Hoffmann, T.; Russe, E.; Scheiblhofer, S.; Weiss, R.; et al. Evaluation of modified Interferon alpha mRNA constructs for the treatment of non-melanoma skin cancer. Sci. Rep. 2018, 8, 12954. [Google Scholar] [CrossRef] [PubMed]
- Firdessa-Fite, R.; Creusot, R.J. Nanoparticles versus Dendritic Cells as Vehicles to Deliver mRNA Encoding Multiple Epitopes for Immunotherapy. Mol. Ther. Methods Clin. Dev. 2020, 16, 50–62. [Google Scholar] [CrossRef] [Green Version]
- WHO. Intradermal delivery of vaccines: Potential benefits and current challenges. Bull. World Health Organ. 2011, 89, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Oosterhuis-Kafeja, F.; Van der Wielen, M.; Almagor, Y.; Sharon, O.; Levin, Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine 2009, 27, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Golombek, S.; Pilz, M.; Steinle, H.; Kochba, E.; Levin, Y.; Lunter, D.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Intradermal Delivery of Synthetic mRNA Using Hollow Microneedles for Efficient and Rapid Production of Exogenous Proteins in Skin. Mol. Ther. Nucleic Acids 2018, 11, 382–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratieri, T.; Alberti, I.; Lapteva, M.; Kalia, Y.N. Next generation intra- and transdermal therapeutic systems: Using non- and minimally-invasive technologies to increase drug delivery into and across the skin. Eur. J. Pharm. Sci. 2013, 50, 609–622. [Google Scholar] [CrossRef]
- Agius, E.; Mooney, J.M.; Bezzina, A.C.; Yu, R.C. Dermojet delivery of bleomycin for the treatment of recalcitrant plantar warts. J. Dermatolog. Treat. 2006, 17, 112–116. [Google Scholar] [CrossRef]
- Bachhav, Y.G.; Summer, S.; Heinrich, A.; Bragagna, T.; Böhler, C.; Kalia, Y.N. Effect of controlled laser microporation on drug transport kinetics into and across the skin. J. Control. Release 2010, 146, 31–36. [Google Scholar] [CrossRef]
- Yu, J.; Kalaria, D.R.; Kalia, Y.N. Erbium: YAG fractional laser ablation for the percutaneous delivery of intact functional therapeutic antibodies. J. Control. Release 2011, 156, 53–59. [Google Scholar] [CrossRef]
- Yu, J.; Dubey, S.; Kalia, Y.N. Needle-free cutaneous delivery of living human cells by Er: YAG fractional laser ablation. Expert Opin. Drug Deliv. 2018, 15, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, M.; Del Río-Sancho, S.; Wu, E.; Carbonell, W.S.; Böhler, C.; Kalia, Y.N. Fractional laser ablation for the targeted cutaneous delivery of an anti-CD29 monoclonal antibody—OS2966. Sci. Rep. 2019, 9, 1030. [Google Scholar] [CrossRef] [Green Version]
- Bauer, M.; Lackner, E.; Matzneller, P.; Al Jalali, V.; Pajenda, S.; Ling, V.; Böhler, C.; Braun, W.; Braun, R.; Boesch, M.; et al. Phase I Study to Assess Safety of Laser-Assisted Topical Administration of an Anti-TNF Biologic in Patients with Chronic Plaque-Type Psoriasis. Front. Med. 2021, 8, 712511. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.S.; McCullough, J.L.; Glenn, T.C.; Wright, W.H.; Liaw, L.H.; Jacques, S.L. Mid-infrared laser ablation of stratum corneum enhances in vitro percutaneous transport of drugs. J. Investig. Dermatol. 1991, 97, 874–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quartier, J.; Capony, N.; Lapteva, M.; Kalia, Y.N. Cutaneous Biodistribution: A High-Resolution Methodology to Assess Bioequivalence in Topical Skin Delivery. Pharmaceutics 2019, 11, 484. [Google Scholar] [CrossRef] [Green Version]
- Lapteva, M.; Mondon, K.; Möller, M.; Gurny, R.; Kalia, Y.N. Polymeric micelle nanocarriers for the cutaneous delivery of tacrolimus: A targeted approach for the treatment of psoriasis. Mol. Pharm. 2014, 11, 2989–3001. [Google Scholar] [CrossRef]
- Tan, K.T.; McGrouther, D.A.; Day, A.J.; Milner, C.M.; Bayat, A. Characterization of hyaluronan and TSG-6 in skin scarring: Differential distribution in keloid scars, normal scars and unscarred skin. J. Eur. Acad. Dermatol. Venereol. JEADV 2011, 25, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Uren, R.F. Lymphatic drainage of the skin. Ann. Surg. Oncol. 2004, 11, 179S–185S. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.A. Erbium: YAG laser. J. Ästhetische Chir. 2020, 13, 100–110. [Google Scholar] [CrossRef]
- Taudorf, E.H.; Lerche, C.M.; Erlendsson, A.M.; Philipsen, P.A.; Hansen, S.H.; Janfelt, C.; Paasch, U.; Anderson, R.R.; Haedersdal, M. Fractional laser-assisted drug delivery: Laser channel depth influences biodistribution and skin deposition of methotrexate. Lasers Surg. Med. 2016, 48, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Machado, Y.; Duinkerken, S.; Hoepflinger, V.; Mayr, M.; Korotchenko, E.; Kurtaj, A.; Pablos, I.; Steiner, M.; Stoecklinger, A.; Lübbers, J.; et al. Synergistic effects of dendritic cell targeting and laser-microporation on enhancing epicutaneous skin vaccination efficacy. J. Control. Release 2017, 266, 87–99. [Google Scholar] [CrossRef]
- del Río-Sancho, S.; Lapteva, M.; Sonaje, K.; Böhler, C.; Ling, V.; Boehncke, W.-H.; Kalia, Y.N. Targeted cutaneous delivery of etanercept using Er: YAG fractional laser ablation. Int. J. Pharm. 2020, 580, 119234. [Google Scholar] [CrossRef] [PubMed]
- Pehrsson, S.; Hölttä, M.; Linhardt, G.; Danielson, R.F.; Carlsson, L. Rapid Production of Human VEGF-A following Intradermal Injection of Modified VEGF-A mRNA Demonstrated by Cutaneous Microdialysis in the Rabbit and Pig In Vivo. BioMed Res. Int. 2019, 2019, 3915851. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Lin, Y.K.; Alalaiwe, A.; Wang, P.W.; Liu, P.Y.; Fang, J.Y. Fractional Laser-Mediated siRNA Delivery for Mitigating Psoriasis-like Lesions via IL-6 Silencing. Mol. Ther. Nucleic Acids 2020, 19, 240–251. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Banga, A.K. Delivery of Methotrexate and Characterization of Skin Treated by Fabricated PLGA Microneedles and Fractional Ablative Laser. Pharm. Res. 2018, 35, 68. [Google Scholar] [CrossRef]
- Sapra, B.; Jindal, M.; Tiwary, A.K. Tight junctions in skin: New perspectives. Ther. Deliv. 2012, 3, 1297–1327. [Google Scholar] [CrossRef]
- Ibaraki, H.; Kanazawa, T.; Takashima, Y.; Okada, H.; Seta, Y. Development of an Innovative Intradermal siRNA Delivery System Using a Combination of a Functional Stearylated Cytoplasm-Responsive Peptide and a Tight Junction-Opening Peptide. Molecules 2016, 21, 1279. [Google Scholar] [CrossRef]








| mRNA | Cy5 Labeled mRNA/eGFP Expressing mRNA | ||
| mRNA transfection agents | Liposomal/Polymeric | ||
| Delivery conditions | System | [Cy5 mRNA] | [eGFP expressing mRNA] |
| ID injection | 1 µg/30 µL 3 µg/30 µL | 1 µg/30 µL 3 µg/30 µL | |
| Hollow microneedle: MicronJet™600 | 1 µg/100 µL | 1 µg/100 µL | |
| Jet injector: Dermojet® (1- and 3-nozzle) | 1 µg/100 µL | 1 µg/100 µL | |
| Fractional laser ablation: Er:YAG (P.L.E.A.SE.) * | 3 µg/30 µL | 3 µg/30 µL | |
| Evaluations | Biodistribution of Cy5 mRNA visualized by CLSM | ||
| Biodistribution of expressed eGFP visualized by CLSM | |||
| Delivery Technique | Delivery Site | Types of Cells Expressing eGFP | |||
|---|---|---|---|---|---|
| Keratinocytes | Fibroblasts | Vascular Endothelium | Appendageal Epithelium | ||
| Intradermal injection | Dermis | +++ | + | ++ | +++ |
| Hollow microneedle injection (MicronJet™600) | Dermis | +++ | + | + | +++ |
| Jet injector | Dermis | +++ | + | + | ++ |
| Fractional laser ablation | Epidermis | + | NA | ++ | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darade, A.R.; Lapteva, M.; Hoffmann, T.; Mandler, M.; Schneeberger, A.; Kalia, Y.N. Effect of mRNA Delivery Modality and Formulation on Cutaneous mRNA Distribution and Downstream eGFP Expression. Pharmaceutics 2022, 14, 151. https://doi.org/10.3390/pharmaceutics14010151
Darade AR, Lapteva M, Hoffmann T, Mandler M, Schneeberger A, Kalia YN. Effect of mRNA Delivery Modality and Formulation on Cutaneous mRNA Distribution and Downstream eGFP Expression. Pharmaceutics. 2022; 14(1):151. https://doi.org/10.3390/pharmaceutics14010151
Chicago/Turabian StyleDarade, Aditya R., Maria Lapteva, Thomas Hoffmann, Markus Mandler, Achim Schneeberger, and Yogeshvar N. Kalia. 2022. "Effect of mRNA Delivery Modality and Formulation on Cutaneous mRNA Distribution and Downstream eGFP Expression" Pharmaceutics 14, no. 1: 151. https://doi.org/10.3390/pharmaceutics14010151
APA StyleDarade, A. R., Lapteva, M., Hoffmann, T., Mandler, M., Schneeberger, A., & Kalia, Y. N. (2022). Effect of mRNA Delivery Modality and Formulation on Cutaneous mRNA Distribution and Downstream eGFP Expression. Pharmaceutics, 14(1), 151. https://doi.org/10.3390/pharmaceutics14010151