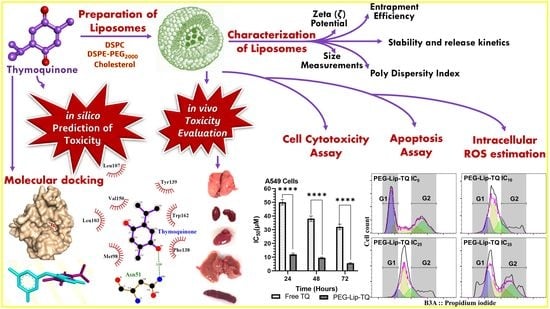
Safety, Stability, and Therapeutic Efficacy of Long-Circulating TQ-Incorporated Liposomes: Implication in the Treatment of Lung Cancer
Abstract
:1. Introduction
2. Methods
2.1. Reagents
2.2. Preparation of Thymoquinone Entrapped Liposomes
2.3. Molecular Docking Studies
2.4. Characterization of Liposomes
2.4.1. The Size, Zeta (ζ) Potential (mV), and Poly Dispersity Index (PDI) and Entrapment Efficiency (EE) of TQ Containing and Empty Liposomes
2.4.2. In Vitro Stability of Liposomes and Release Kinetics of TQ
2.5. Cell Cytotoxicity Assay
2.6. Cell Cycle Distribution Analysis
2.7. Toxicity Study of TQ
2.7.1. In Silico Oral Toxicity Assay
2.7.2. In Vivo Oral Acute Toxicity Assay
2.7.3. Assessment of Relative Organ Weight
2.7.4. Hematological Analyses
2.8. Statistical Analysis
3. Results
3.1. Identification of Predicted Anticancer Targets of TQ by Molecular Docking Studies
3.2. Characterization of Liposomes
3.2.1. Size, PDI, ζ Potential, and EE
3.2.2. In Vitro Stability of Liposomes and Release Kinetics of TQ
3.3. Effect of TQ on Cellular Proliferation and IC50 at Varying Doses of Free TQ and Lip-TQ in A549 and H460 Lung Cancer Cell Lines
3.4. Effect of PEG-Lip-TQ on Cell Cycle Distribution at IC10, IC25, and IC35 in A549 and H460 Lung Cancer Cell Lines
3.5. In Vivo Oral Toxicity Study of TQ
3.5.1. In Situ Toxicity Assay
3.5.2. In Vivo Oral Toxicity Assay in Swiss Albino Mice
General Appearance, Behavioral Observations and LD50
Effect of TQ on Leukocytes by Hematological Studies
Effect of TQ on Relative Organ Weight
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Hameed, R.; Khan, A.; Khan, S.; Perveen, S. Computational Approaches Towards Kinases as Attractive Targets for Anticancer Drug Discovery and Development. Anti Cancer Agents Med. Chem. 2019, 19, 592–598. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [Green Version]
- Chopra, B.; Dhingra, A.K. Natural products: A lead for drug discovery and development. Phytother. Res. 2021, 35, 4660–4702. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, J.; Kong, L.; Yuan, T.; Li, W.; Zhang, W.; Hou, B.; Lu, Y.; Du, G. The strategies and techniques of drug discovery from natural products. Pharmacol. Ther. 2020, 216, 107686. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, M.; Zhao, R.; Wang, D.; Ma, Y.; Li, A. Plant Natural Products: Promising Resources for Cancer Chemoprevention. Molecules 2021, 26, 933. [Google Scholar] [CrossRef] [PubMed]
- Block, G.; Patterson, B.; Subar, A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer 1992, 18, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Singh, A.; Negi, P.; Kapoor, V.K. Thymoquinone: A small molecule from nature with high therapeutic potential. Drug Discov. Today 2021, 26, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.; Roessner, A.; Schneider-Stock, R. Thymoquinone: A promising anti-cancer drug from natural sources. Int. J. Biochem. Cell Biol. 2006, 38, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Younus, H. Thymoquinone Shows the Diverse Therapeutic Actions by Modulating Multiple Cell Signaling Pathways: Single Drug for Multiple Targets. Curr. Pharm. Biotechnol. 2019, 19, 934–945. [Google Scholar] [CrossRef]
- Khader, M.; Eckl, P.M. Thymoquinone: An emerging natural drug with a wide range of medical applications. Iran. J. Basic Med. Sci. 2014, 17, 950–957. [Google Scholar]
- Botnick, I.; Xue, W.; Bar, E.; Ibdah, M.; Schwartz, A.; Joel, D.M.; Lev, E.; Fait, A.; Lewinsohn, E. Distribution of Primary and Specialized Metabolites in Nigella sativa Seeds, a Spice with Vast Traditional and Historical Uses. Molecules 2012, 17, 10159–10177. [Google Scholar] [CrossRef] [PubMed]
- Ansary, J.; Giampieri, F.; Forbes-Hernandez, T.Y.; Regolo, L.; Quinzi, D.; Villar, S.G.; Villena, E.G.; Pifarre, K.T.; Alvarez-Suarez, J.M.; Battino, M.; et al. Nutritional Value and Preventive Role of Nigella sativa L. and Its Main Component Thymoquinone in Cancer: An Evidenced-Based Review of Preclinical and Clinical Studies. Molecules 2021, 26, 2108. [Google Scholar] [CrossRef]
- Khan, A.; Tania, M.; Fu, S.; Fu, J. Thymoquinone, as an anticancer molecule: From basic research to clinical investigation. Oncotarget 2017, 8, 51907–51919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathore, C.; Rathbone, M.J.; Chellappan, D.K.; Tambuwala, M.M.; Pinto, T.D.J.A.; Dureja, H.; Hemrajani, C.; Gupta, G.; Dua, K.; Negi, P. Nanocarriers: More than tour de force for thymoquinone. Expert Opin. Drug Deliv. 2020, 17, 479–494. [Google Scholar] [CrossRef]
- Salmani, J.M.M.; Asghar, S.; Lv, H.; Zhou, J. Aqueous Solubility and Degradation Kinetics of the Phytochemical Anticancer Thymoquinone; Probing the Effects of Solvents, pH and Light. Molecules 2014, 19, 5925–5939. [Google Scholar] [CrossRef] [Green Version]
- Allemailem, K.; Alnuqaydan, A.; Almatroudi, A.; Alrumaihi, F.; Aljaghwani, A.; Khalilullah, H.; Younus, H.; Khan, A.; Khan, M. Safety and Therapeutic Efficacy of Thymoquinone-Loaded Liposomes against Drug-Sensitive and Drug-Resistant Acinetobacter baumannii. Pharmaceutics 2021, 13, 677. [Google Scholar] [CrossRef]
- Alam Khan, M.; Aljarbou, A.N.; Khan, A.; Younus, H. Liposomal thymoquinone effectively combats fluconazole-resistant Candida albicans in a murine model. Int. J. Biol. Macromol. 2015, 76, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Odeh, F.; Ismail, S.I.; Abu-Dahab, R.; Mahmoud, I.; Al Bawab, A. Thymoquinone in liposomes: A study of loading efficiency and biological activity towards breast cancer. Drug Deliv. 2012, 19, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Odeh, F.; Naffa, R.; Azzam, H.; Mahmoud, I.; Alshaer, W.; Al Bawab, A.; Ismail, S. Co-encapsulation of thymoquinone with docetaxel enhances the encapsulation efficiency into PEGylated liposomes and the chemosensitivity of MCF7 breast cancer cells to docetaxel. Heliyon 2019, 5, e02919. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, N.; Mehrabian, A.; Amiri-Darban, S.; Moosavian, S.A.; Jaafari, M.R. Preparation and characterization of PEGylated liposomal Doxorubicin targeted with leptin-derived peptide and evaluation of their anti-tumor effects, in vitro and in vivo in mice bearing C26 colon carcinoma. Colloids Surfaces B Biointerfaces 2021, 200, 111589. [Google Scholar] [CrossRef]
- Pozzi, D.; Colapicchioni, V.; Caracciolo, G.; Piovesana, S.; Capriotti, A.L.; Palchetti, S.; De Grossi, S.; Riccioli, A.; Amenitsch, H.; Laganà, A. Effect of polyethyleneglycol (PEG) chain length on the bio–nano-interactions between PEGylated lipid nanoparticles and biological fluids: From nanostructure to uptake in cancer cells. Nanoscale 2014, 6, 2782–2792. [Google Scholar] [CrossRef] [PubMed]
- Chow, T.-H.; Lin, Y.-Y.; Hwang, J.-J.; Wang, H.-E.; Tseng, Y.-L.; Wang, S.-J.; Liu, R.S.; Lin, W.J.; Yang, C.S.; Ting, G. Improvement of biodistribution and therapeutic index via increase of polyethylene glycol on drug-carrying liposomes in an HT-29/luc xenografted mouse model. Anticancer. Res. 2009, 9, 2111–2120. [Google Scholar]
- Fatima, N.; Nayeem, N. Toxic Effects as a Result of Herbal Medicine Intake. In Toxicology—New Aspects to This Scientific Conundrum; InTech Open: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Onakpoya, I.J.; Posadzki, P.; Eddouks, M. The Safety of Herbal Medicine: From Prejudice to Evidence. Evid. Based Complement. Altern. Med. 2015, 2015, 316706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousseaux, C.G.; Schachter, H. Regulatory issues concerning the safety, efficacy and quality of herbal remedies. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2003, 68, 505–510. [Google Scholar] [CrossRef] [PubMed]
- OECD Test No. 452: Chronic Toxicity Studies; OECD: Paris, France, 2018. [CrossRef]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef] [PubMed]
- OECD Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure; OECD: Paris, France, 2008. [CrossRef] [Green Version]
- Tang, J.; Guo, F.; Ding, Y. The Computational Models of Drug-target Interaction Prediction. Protein Pept. Lett. 2020, 27, 348–358. [Google Scholar] [CrossRef]
- Ferrero, E.; Dunham, I.; Sanseau, P. In silico prediction of novel therapeutic targets using gene–disease association data. J. Transl. Med. 2017, 15, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanzifi, M.; Yaraki, M.T.; Beiramzadeh, Z.; Saremi, L.H.; Najafifard, M.; Moradi, H.; Mansouri, M.; Karami, M.; Bazgir, H. Carboxymethyl cellulose improved adsorption capacity of polypyrrole/CMC composite nanoparticles for removal of reactive dyes: Experimental optimization and DFT calculation. Chemosphere 2020, 255, 127052. [Google Scholar] [CrossRef]
- Azam, F.; Abodabos, H.S.; Taban, I.M.; Rfieda, A.R.; Mahmood, D.; Anwar, J.; Khan, S.; Sizochenko, N.; Poli, G.; Tuccinardi, T.; et al. Rutin as promising drug for the treatment of Parkinson’s disease: An assessment of MAO-B inhibitory potential by docking, molecular dynamics and DFT studies. Mol. Simul. 2019, 45, 1563–1571. [Google Scholar] [CrossRef]
- Khan, A.; Aljarbou, A.N.; Aldebasi, Y.H.; Allemeilam, K.S.; A Alsahly, M.; Khan, S.; Alruwetei, A.M.; A Khan, M. Fatty Acid Synthase (FASN) siRNA-Encapsulated-Her-2 Targeted Fab’-Immunoliposomes for Gene Silencing in Breast Cancer Cells. Int. J. Nanomed. 2020, 15, 5575–5589. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef] [Green Version]
- Azam, F. Elucidation of Teicoplanin Interactions with Drug Targets Related to COVID-19. Antibiotics 2021, 10, 856. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Al-Sayed, E.; Moghannem, S.; Azam, F.; El-Shazly, M.; Singab, A.N. Breaking Down the Barriers to a Natural Antiviral Agent: Antiviral Activity and Molecular Docking of Erythrina speciosa Extract, Fractions, and the Major Compound. Chem. Biodivers. 2020, 17, e1900511. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Aldebasi, Y.H.; Alsuhaibani, S.A.; Alsahli, M.A.; Alzohairy, M.A.; Khan, A.; Younus, H. Therapeutic potential of thymoquinone liposomes against the systemic infection of Candida albicans in diabetic mice. PLoS ONE 2018, 13, e0208951. [Google Scholar] [CrossRef]
- Banerjee, P.; O Eckert, A.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [Green Version]
- Martin, T. Toxicity Estimation Software Tool (TEST). 2016. Available online: https://www.epa.gov/chemical-research/toxicity-estimation-software-tool-test (accessed on 3 January 2022).
- Hussain, S.; Azam, F.; Eldarrat, H.A.; Alkskas, I.; Mayoof, J.A.; Dammona, J.M.; Ismail, H.; Ali, M.; Arif, M.; Haque, A. Anti-inflammatory, analgesic and molecular docking studies of Lanostanoic acid 3-O-α-D-glycopyranoside isolated from Helichrysum stoechas. Arab. J. Chem. 2020, 13, 9196–9206. [Google Scholar] [CrossRef]
- Jeon, J.; Kang, S.; Kim, H.U. Predicting biochemical and physiological effects of natural products from molecular structures using machine learning. Nat. Prod. Rep. 2021, 38, 1954–1966. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2018, 27, 742–761. [Google Scholar] [CrossRef]
- Anderson, M.; Omri, A. The Effect of Different Lipid Components on the In Vitro Stability and Release Kinetics of Liposome Formulations. Drug Deliv. 2004, 11, 33–39. [Google Scholar] [CrossRef]
- Crommelin, D.J. Influence of Lipid Composition and Ionic Strength on the Physical Stability of Liposomes. J. Pharm. Sci. 1984, 73, 1559–1563. [Google Scholar] [CrossRef]
- Briuglia, M.-L.; Rotella, C.M.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Magarkar, A.; Dhawan, V.; Kallinteri, P.; Viitala, T.; Elmowafy, M.; Róg, T.; Bunker, A. Cholesterol level affects surface charge of lipid membranes in saline solution. Sci. Rep. 2014, 4, 5005. [Google Scholar] [CrossRef] [Green Version]
- Papahadjopoulos, D.; Cowden, M.; Kimelberg, H. Role of cholesterol in membranes effects on phospholipid-protein interactions, membrane permeability and enzymatic activity. Biochim. Biophys. Acta (BBA) Biomembr. 1973, 330, 8–26. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef]
- Singh, A.; Neupane, Y.R.; Shafi, S.; Mangla, B.; Kohli, K. PEGylated liposomes as an emerging therapeutic platform for oral nanomedicine in cancer therapy: In vitro and in vivo assessment. J. Mol. Liq. 2020, 303, 112649. [Google Scholar] [CrossRef]
- Jiménez-López, J.; Bravo-Caparrós, I.; Cabeza, L.; Nieto, F.R.; Ortiz, R.; Perazzoli, G.; Fernández-Segura, E.; Cañizares, F.J.; Baeyens, J.M.; Melguizo, C.; et al. Paclitaxel antitumor effect improvement in lung cancer and prevention of the painful neuropathy using large pegylated cationic liposomes. Biomed. Pharmacother. 2021, 133, 111059. [Google Scholar] [CrossRef]
- Mostafa, M.; Alaaeldin, E.; Aly, U.F.; Sarhan, H.A. Optimization and Characterization of Thymoquinone-Loaded Liposomes with Enhanced Topical Anti-inflammatory Activity. AAPS PharmSciTech 2018, 19, 3490–3500. [Google Scholar] [CrossRef]
- Hernández-Alvarado, R.B.; Madariaga-Mazón, A.; Martinez-Mayorga, K. Prediction of toxicity of secondary metabolites. Phys. Sci. Rev. 2019, 4. [Google Scholar] [CrossRef]
- Madariaga-Mazón, A.; Hernández-Alvarado, R.B.; Noriega-Colima, K.O.; Osnaya-Hernández, A.; Martinez-Mayorga, K. Toxicity of secondary metabolites. Phys. Sci. Rev. 2019, 4. [Google Scholar] [CrossRef]
- Raies, A.; Bajic, V.B. In silicotoxicology: Computational methods for the prediction of chemical toxicity. WIREs Comput. Mol. Sci. 2016, 6, 147–172. [Google Scholar] [CrossRef] [Green Version]
- Badary, O.; Al-Shabanah, O.A.; Nagi, M.N.; Al-Bekairi, A.M.; Elmazar, M.M. Acute and subchronic toxicity of thymoquinone in mice. Drug Dev. Res. 1998, 44, 56–61. [Google Scholar] [CrossRef]
- Moser, C.; Lang, S.A.; Stoeltzing, O. Heat-shock protein 90 (Hsp90) as a molecular target for therapy of gastrointestinal cancer. Anticancer Res. 2009, 29, 2031–2042. [Google Scholar] [PubMed]
- Pantsar, T.; Poso, A. Binding Affinity via Docking: Fact and Fiction. Molecules 2018, 23, 1899. [Google Scholar] [CrossRef] [Green Version]














| Phases | Free TQ (mg/kg/b.w) | PEG-Lip-TQ (mg/kg/b.w) |
|---|---|---|
| Phase-1 | 0, 10, 100, 1000 | 0, 10, 100, 1000 |
| Phase-2 | 0, 20, 40, 80, 100, 160 | 0, 600, 1000, 1600, 2900 |
| S.N | Classification | Target | Prediction | Probability |
|---|---|---|---|---|
| 1. | Organ toxicity | Hepatotoxicity | Inactive | 0.63 |
| 2. | Toxicity end points | Carcinogenicity | Inactive | 0.63 |
| Immunotoxicity | Inactive | 0.97 | ||
| Mutagenicity | Inactive | 0.91 | ||
| Cytotoxicity | Inactive | 0.78 | ||
| 3. | Nuclear receptor signaling pathways | Aryl hydrocarbon receptor | Inactive | 0.93 |
| Androgen receptor | Inactive | 0.99 | ||
| Androgen receptor ligand binding domain | Inactive | 0.99 | ||
| Aromatase | Inactive | 0.99 | ||
| Estrogen receptor alpha | Inactive | 0.96 | ||
| Estrogen receptor ligand binding domain | Inactive | 0.93 | ||
| Peroxisome proliferator activated receptor gamma | Inactive | 0.99 | ||
| 4. | Stress response pathways | Nuclear factor (erythroid-derived 2)-like 2/antioxidant responsive element (nrf2/ARE) | Inactive | 0.93 |
| Heat shock factor response element (HSE) | Inactive | 0.93 | ||
| Mitochondrial membrane potential | Active | 0.58 | ||
| Phosphoprotein (tumor suppressor) p53 | Inactive | 0.92 | ||
| ATPase family AAA domain-containing protein 5 (ATAD5) | Inactive | 0.99 |
| Toxicity Parameters | Toxicity Results | |
|---|---|---|
| Consensus method | Oral rat LD50 mg/kg | 816.9 |
| Mutagenicity value | 0.19 | |
| Mutagenicity result | Negative | |
| Hierarchical clustering method | Oral rat LD50 mg/kg | 1004.5 |
| Mutagenicity value | 0.05 | |
| Mutagenicity result | Negative | |
| Nearest neighbor method | Oral rat LD50 mg/kg | 664.4 |
| Mutagenicity value | 0.33 | |
| Mutagenicity result | Negative | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Alsahli, M.A.; Aljasir, M.A.; Maswadeh, H.; Mobark, M.A.; Azam, F.; Allemailem, K.S.; Alrumaihi, F.; Alhumaydhi, F.A.; Alwashmi, A.S.S.; et al. Safety, Stability, and Therapeutic Efficacy of Long-Circulating TQ-Incorporated Liposomes: Implication in the Treatment of Lung Cancer. Pharmaceutics 2022, 14, 153. https://doi.org/10.3390/pharmaceutics14010153
Khan A, Alsahli MA, Aljasir MA, Maswadeh H, Mobark MA, Azam F, Allemailem KS, Alrumaihi F, Alhumaydhi FA, Alwashmi ASS, et al. Safety, Stability, and Therapeutic Efficacy of Long-Circulating TQ-Incorporated Liposomes: Implication in the Treatment of Lung Cancer. Pharmaceutics. 2022; 14(1):153. https://doi.org/10.3390/pharmaceutics14010153
Chicago/Turabian StyleKhan, Arif, Mohammed A. Alsahli, Mohammad A. Aljasir, Hamzah Maswadeh, Mugahid A. Mobark, Faizul Azam, Khaled S. Allemailem, Faris Alrumaihi, Fahad A. Alhumaydhi, Ameen S. S. Alwashmi, and et al. 2022. "Safety, Stability, and Therapeutic Efficacy of Long-Circulating TQ-Incorporated Liposomes: Implication in the Treatment of Lung Cancer" Pharmaceutics 14, no. 1: 153. https://doi.org/10.3390/pharmaceutics14010153
APA StyleKhan, A., Alsahli, M. A., Aljasir, M. A., Maswadeh, H., Mobark, M. A., Azam, F., Allemailem, K. S., Alrumaihi, F., Alhumaydhi, F. A., Alwashmi, A. S. S., Almatroudi, A. A., Alsugoor, M. H., & Khan, M. A. (2022). Safety, Stability, and Therapeutic Efficacy of Long-Circulating TQ-Incorporated Liposomes: Implication in the Treatment of Lung Cancer. Pharmaceutics, 14(1), 153. https://doi.org/10.3390/pharmaceutics14010153