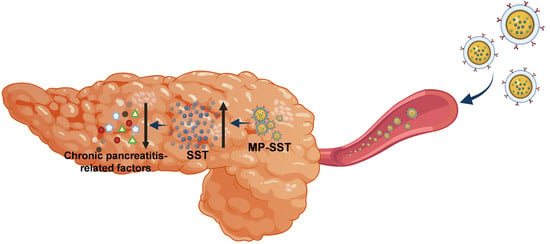
A Macrophage Membrane–Polymer Hybrid Biomimetic Nanoplatform for Therapeutic Delivery of Somatostatin Peptide to Chronic Pancreatitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mateirals
2.2. Fabrication of Nanoparticles
2.3. Characterization of Nanoparticles
2.4. Encapsulation and Loading Efficiency
2.5. Cumulative Release
2.6. SDS-PAGE
2.7. Biocompatability In Vitro
2.8. Cellular Uptake
2.9. The Administration of Nanoparticles to Mice with Chronic Pancreatitis
2.10. ELISA
2.11. Hematoxylin–Eosin and Masson’s Trichrome Staining
2.12. Statistical Analysis
3. Results and Discussion
3.1. Fabrication and Characterization of Nanoparticles
3.2. Biocompatibility and Cellular Uptake of Nanoparticles In Vitro
3.3. Repair of Chronic Pancreatitis by Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleeff, J.; Whitcomb, D.C.; Shimosegawa, T.; Esposito, I.; Lerch, M.M.; Gress, T.; Mayerle, J.; Drewes, A.M.; Rebours, V.; Akisik, F.; et al. Chronic pancreatitis. Nat. Rev. Dis. Primers 2017, 3, 17060. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.B.; Adler, D.G.; Forsmark, C.E.; Sauer, B.G.; Taylor, J.R.; Whitcomb, D.C. ACG clinical guideline: Chronic pancreatitis. Am. J. Gastroenterol. 2020, 115, 322–339. [Google Scholar] [CrossRef] [PubMed]
- Beyer, G.; Habtezion, A.; Werner, J.; Lerch, M.M.; Mayerle, J. Chronic pancreatitis. Lancet 2020, 396, 499–512. [Google Scholar] [CrossRef]
- Hart, P.A.; Conwell, D.L. Chronic pancreatitis: Managing a difficult disease. Am. J. Gastroenterol. 2020, 115, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Büchler, M.W.; Binder, M.; Friess, H. Role of somatostatin and its analogues in the treatment of acute and chronic pancreatitis. Gut 1994, 35, S15–S19. [Google Scholar] [CrossRef]
- Burgus, R.; Ling, N.; Butcher, M.; Guillemin, R. Primary structure of somatostatin, a hypothalamic peptide that inhibits the secretion of pituitary growth hormone. Proc. Natl. Acad. Sci. USA 1973, 70, 684–688. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Owyang, C. Somatostatin inhibits pancreatic enzyme secretion at a central vagal site. Am. J. Physiol. Gastrointest. Liver Physiol. 1993, 265, G251–G257. [Google Scholar] [CrossRef]
- Śliwińska-Mossoń, M.; Veselý, M.; Milnerowicz, H. The clinical significance of somatostatin in pancreatic diseases. Ann. Endocrinol. 2014, 75, 232–240. [Google Scholar] [CrossRef]
- Rai, U.; Thrimawithana, T.R.; Valery, C.; Young, S.A. Therapeutic uses of somatostatin and its analogues: Current view and potential applications. Pharmacol. Ther. 2015, 152, 98–110. [Google Scholar] [CrossRef]
- Bruns, C.; Lewis, I.; Briner, U.; Meno-Tetang, G.; Weckbecker, G. SOM230: A novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur. J. Endocrinol. 2002, 146, 707–716. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Miller, T.E.; Prasad, P.; Lee, J.; Krauss, J.; Miscik, K.; Kalafsky, G.; McLeod, J.E. Pharmacokinetics, pharmacodynamics, and safety of microencapsulated octreotide acetate in healthy subjects. J. Clin. Pharmacol. 2000, 40, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Tomlinson, B. Pharmacokinetic evaluation of lanreotide. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Schmid, H.A. Pasireotide (SOM230): Development, mechanism of action and potential applications. Mol. Cell Endocrinol. 2008, 286, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Fattah, S.; Brayden, D.J. Progress in the formulation and delivery of somatostatin analogs for acromegaly. Ther. Deliv. 2017, 8, 867–878. [Google Scholar] [CrossRef] [Green Version]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Yan, W.L.; Lang, T.Q.; Yuan, W.H.; Yin, Q.; Li, Y.P. Nanosized drug delivery systems modulate the immunosuppressive microenvironment to improve cancer immunotherapy. Acta Pharmacol. Sin. 2022, 1–10. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [Green Version]
- Acar, H.; Ting, J.M.; Srivastava, S.; LaBelle, J.L.; Tirrell, M.V. Molecular engineering solutions for therapeutic peptide delivery. Chem. Soc. Rev. 2017, 46, 6553–6569. [Google Scholar] [CrossRef]
- Chellappan, D.K.; Prasher, P.; Saravanan, V.; Vern Yee, V.S.; Wen Chi, W.C.; Wong, J.W.; Wong, J.K.; Wong, J.T.; Wan, W.; Chellian, J.; et al. Protein and peptide delivery to lungs by using advanced targeted drug delivery. Chem. Biol. Interact. 2022, 351, 109706. [Google Scholar] [CrossRef]
- Mun, S.-J.; Cho, E.; Kim, J.-S.; Yang, C.-S. Pathogen-derived peptides in drug targeting and its therapeutic approach. J. Control. Release 2022, 350, 716–733. [Google Scholar] [CrossRef] [PubMed]
- Öberg, K.; Lamberts, S.W.J. Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: Past, present and future. Endocr. Relat. Cancer 2016, 23, R551–R566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, N.; Varshney, R.; Shukla, J.; Ganeshpurkar, A.; Hazari, P.P.; Bandopadhaya, G.P.; Mishra, A.K.; Trivedi, P. Synthesis and evaluation of biodegradable PCL/PEG nanoparticles for neuroendocrine tumor targeted delivery of somatostatin analog. Drug Deliv. 2012, 19, 132–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, R.; Giannaccini, M.; Dal Monte, M.; Cammalleri, M.; Pini, A.; Raffa, V.; Lulli, M.; Casini, G. Association of the somatostatin analog octreotide with magnetic nanoparticles for intraocular delivery: A possible approach for the treatment of diabetic retinopathy. Front. Bioeng. Biotechnol. 2020, 8, 144. [Google Scholar] [CrossRef]
- Larocca, A.V.; Toniolo, G.; Tortorella, S.; Krokidis, M.G.; Menounou, G.; Di Bella, G.; Chatgilialoglu, C.; Ferreri, C. The entrapment of somatostatin in a lipid formulation: Retarded release and free radical reactivity. Molecules 2019, 24, 3085. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Mu, H. Lipid and PLGA microparticles for sustained delivery of protein and peptide drugs. Pharm. Nanotechnol. 2020, 8, 22–32. [Google Scholar] [CrossRef]
- Dodda, J.M.; Remiš, T.; Rotimi, S.; Yeh, Y.-C. Progress in the drug encapsulation of poly(lactic-co-glycolic acid) and folate-decorated poly(ethylene glycol)–poly(lactic-co-glycolic acid) conjugates for selective cancer treatment. J. Mat. Chem. B 2022, 10, 4127–4141. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(lactic acid)-based microparticles for drug delivery applications: An overview of recent advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef]
- Bose, R.J.C.; Lee, S.-H.; Park, H. Lipid-based surface engineering of PLGA nanoparticles for drug and gene delivery applications. Biomater. Res. 2016, 20, 34. [Google Scholar] [CrossRef] [Green Version]
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Arias, J.L. Recent advances in the surface functionalization of PLGA-based nanomedicines. Nanomaterials 2022, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- Zalba, S.; Ten Hagen, T.L.M.; Burgui, C.; Garrido, M.J. Stealth nanoparticles in oncology: Facing the PEG dilemma. J. Control. Release 2022, 351, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Facing the truth about nanotechnology in drug delivery. ACS Nano 2013, 7, 7442–7447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- De Lázaro, I.; Mooney, D.J. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater. 2021, 20, 1469–1479. [Google Scholar] [CrossRef]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell membrane coating nanotechnology. Adv. Mater. 2018, 30, 1706759. [Google Scholar] [CrossRef]
- Zou, D.; Wang, H.; Liu, X.; Xu, Z.P.; Roberts, M.S.; Zhao, C.-X. Artificial cells for the treatment of liver diseases. Acta Biomater. 2021, 130, 98–114. [Google Scholar] [CrossRef]
- Ferreira-Faria, I.; Yousefiasl, S.; Macário-Soares, A.; Pereira-Silva, M.; Peixoto, D.; Zafar, H.; Raza, F.; Faneca, H.; Veiga, F.; Hamblin, M.R.; et al. Stem cell membrane-coated abiotic nanomaterials for biomedical applications. J. Control. Release 2022, 351, 174–197. [Google Scholar] [CrossRef]
- Liu, W.; Huang, Y. Cell membrane-engineered nanoparticles for cancer therapy. J. Mat. Chem. B 2022, 10, 7161–7172. [Google Scholar] [CrossRef]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef]
- Nourshargh, S.; Alon, R. Leukocyte migration into inflamed tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, K.; Li, T.; Maruf, A.; Qin, X.; Luo, L.; Zhong, Y.; Qiu, J.; McGinty, S.; Pontrelli, G.; et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics 2021, 11, 164–180. [Google Scholar] [CrossRef]
- Khatoon, N.; Zhang, Z.; Zhou, C.; Chu, M. Macrophage membrane coated nanoparticles: A biomimetic approach for enhanced and targeted delivery. Biomater. Sci. 2022, 10, 1193–1208. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Zhou, Z.; Bai, D.; Zhang, Q.; Ai, X.; Gao, W.; Zhang, L. White blood cell membrane-coated nanoparticles: Recent development and medical applications. Adv. Healthc. Mater. 2022, 11, 2101349. [Google Scholar] [CrossRef]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.-B.; Lee, S.M.Y.; Wang, R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622. [Google Scholar] [CrossRef]
- Thamphiwatana, S.; Angsantikul, P.; Escajadillo, T.; Zhang, Q.; Olson, J.; Luk, B.T.; Zhang, S.; Fang, R.H.; Gao, W.; Nizet, V.; et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc. Natl. Acad. Sci. USA 2017, 114, 11488–11493. [Google Scholar] [CrossRef] [Green Version]
- Jash, A.; Ubeyitogullari, A.; Rizvi, S.S.H. Liposomes for oral delivery of protein and peptide-based therapeutics: Challenges, formulation strategies, and advances. J. Mat. Chem. B 2021, 9, 4773–4792. [Google Scholar] [CrossRef]
- Lv, S.; Sylvestre, M.; Prossnitz, A.N.; Yang, L.F.; Pun, S.H. Design of polymeric carriers for intracellular peptide delivery in oncology applications. Chem. Rev. 2021, 121, 11653–11698. [Google Scholar] [CrossRef]
- Niu, X.; Zou, W.; Liu, C.; Zhang, N.; Fu, C. Modified nanoprecipitation method to fabricate DNA-loaded PLGA nanoparticles. Drug Dev. Ind. Pharm. 2009, 35, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.S.; Thakur, A.K. Biodegradable delivery system containing a peptide inhibitor of polyglutamine aggregation: A step toward therapeutic development in Huntington’s disease. J. Pept. Sci. 2014, 20, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Qi, G.; Wang, B.; Yu, T.; Zhang, Y.; Li, H.; Kitte, S.A.; Jin, Y. Ultrasound-activated Au/ZnO-based Trojan nanogenerators for combined targeted electro-stimulation and enhanced catalytic therapy of tumor. Nano Energy 2021, 87, 106208. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, R.; Yang, X.; Lu, Y.; Yang, Z.; Peng, M.; Ma, Z.; Jiao, J.; Li, L. A honeycomb-like bismuth/manganese oxide nanoparticle with mutual reinforcement of internal and external response for triple-negative breast cancer targeted therapy. Adv. Healthc. Mater. 2021, 10, 2100518. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Hu, C.-M.J.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef]
- Yoo, H.S.; Choi, H.K.; Park, T.G. Protein–fatty acid complex for enhanced loading and stability within biodegradable nanoparticles. J. Pharm. Sci. 2001, 90, 194–201. [Google Scholar] [CrossRef]
- Matsumura, N.; Ochi, K.; Ichimura, M.; Mizushima, T.; Harada, H.; Harada, M. Study on free radicals and pancreatic fibrosis—Pancreatic fibrosis induced by repeated injections of superoxide dismutase inhibitor. Pancreas 2001, 22, 53–57. [Google Scholar] [CrossRef]
- Li, K.; Yu, M.; Hu, Y.; Ren, G.; Zang, T.; Xu, X.; Qu, J. Three kinds of Ganoderma lucidum polysaccharides attenuate DDC-induced chronic pancreatitis in mice. Chem. Biol. Interact. 2016, 247, 30–38. [Google Scholar] [CrossRef]
- Govender, T.; Stolnik, S.; Garnett, M.C.; Illum, L.; Davis, S.S. PLGA nanoparticles prepared by nanoprecipitation: Drug loading and release studies of a water soluble drug. J. Control. Release 1999, 57, 171–185. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef]
- Park, J.H.; Jiang, Y.; Zhou, J.; Gong, H.; Mohapatra, A.; Heo, J.; Gao, W.; Fang, R.H.; Zhang, L. Genetically engineered cell membrane–coated nanoparticles for targeted delivery of dexamethasone to inflamed lungs. Sci. Adv. 2021, 7, eabf7820. [Google Scholar] [CrossRef] [PubMed]
- Holay, M.; Zhou, J.; Park, J.H.; Landa, I.; Ventura, C.J.; Gao, W.; Fang, R.H.; Zhang, L. Organotropic targeting of biomimetic nanoparticles to treat lung disease. Bioconjug. Chem. 2022, 33, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C.; Frulloni, L.; Garg, P.; Greer, J.B.; Schneider, A.; Yadav, D.; Shimosegawa, T. Chronic pancreatitis: An international draft consensus proposal for a new mechanistic definition. Pancreatology 2016, 16, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Zhang, L.; Miron, R.J.; Liang, J.; Shi, M.; Mo, W.; Zheng, S.; Zhao, Y.; Zhang, Y. Pretreated macrophage-membrane-coated gold nanocages for precise drug delivery for treatment of bacterial infections. Adv. Mater. 2018, 30, 1804023. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ran, D.; Campeau, A.; Xiao, C.; Zhou, J.; Dehaini, D.; Jiang, Y.; Kroll, A.V.; Zhang, Q.; Gao, W.; et al. Multiantigenic nanotoxoids for antivirulence vaccination against antibiotic-resistant gram-negative bacteria. Nano Lett. 2019, 19, 4760–4769. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, J.; Zhou, J.; Fang, R.H.; Gao, W.; Zhang, L. Lure-and-kill macrophage nanoparticles alleviate the severity of experimental acute pancreatitis. Nat. Commun. 2021, 12, 4136. [Google Scholar] [CrossRef]
- Parodi, A.; Quattrocchi, N.; van de Ven, A.L.; Chiappini, C.; Evangelopoulos, M.; Martinez, J.O.; Brown, B.S.; Khaled, S.Z.; Yazdi, I.K.; Enzo, M.V.; et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol. 2013, 8, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Corbo, C.; Molinaro, R.; Taraballi, F.; Toledano Furman, N.E.; Hartman, K.A.; Sherman, M.B.; De Rosa, E.; Kirui, D.K.; Salvatore, F.; Tasciotti, E. Unveiling the in vivo protein corona of circulating leukocyte-like carriers. ACS Nano 2017, 11, 3262–3273. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Strate, T.; Yekebas, E.; Knoefel, W.T.; Bloechle, C.; Izbicki, J.R. Pathogenesis and the natural course of chronic pancreatitis. Eur. J. Gastroenterol. Hepatol. 2002, 14, 929–934. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, F.; Bi, Y.; Ji, B. Animal models of gastrointestinal and liver diseases. Animal models of acute and chronic pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G343–G355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerch, M.M.; Gorelick, F.S. Models of acute and chronic pancreatitis. Gastroenterology 2013, 144, 1180–1193. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sheng, Y.; Yu, M.; Li, K.; Ren, G.; Xu, X.; Qu, J. Antioxidant activity of Inonotus obliquus polysaccharide and its amelioration for chronic pancreatitis in mice. Int. J. Biol. Macromol. 2016, 87, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Kitahashi, T.; Imai, T.; Ishigamori, R.; Takasu, S.; Mutoh, M.; Sugimura, T.; Wakabayashi, K.; Takahashi, M. Enhancement of carcinogenesis and fatty infiltration in the pancreas in N-nitrosobis(2-oxopropyl)amine-treated hamsters by high-fat diet. Pancreas 2011, 40, 1234–1240. [Google Scholar] [CrossRef]
- Hori, M.; Takahashi, M.; Hiraoka, N.; Yamaji, T.; Mutoh, M.; Ishigamori, R.; Furuta, K.; Okusaka, T.; Shimada, K.; Kosuge, T.; et al. Association of pancreatic fatty infiltration with pancreatic ductal adenocarcinoma. Clin. Transl. Gastroenterol. 2014, 5, e53. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Deng, Y.; Yu, L.; Zhou, A.; Wang, J.; Jia, J.; Li, N.; Ding, F.; Lian, W.; Liu, Q.; et al. A Macrophage Membrane–Polymer Hybrid Biomimetic Nanoplatform for Therapeutic Delivery of Somatostatin Peptide to Chronic Pancreatitis. Pharmaceutics 2022, 14, 2341. https://doi.org/10.3390/pharmaceutics14112341
Wang F, Deng Y, Yu L, Zhou A, Wang J, Jia J, Li N, Ding F, Lian W, Liu Q, et al. A Macrophage Membrane–Polymer Hybrid Biomimetic Nanoplatform for Therapeutic Delivery of Somatostatin Peptide to Chronic Pancreatitis. Pharmaceutics. 2022; 14(11):2341. https://doi.org/10.3390/pharmaceutics14112341
Chicago/Turabian StyleWang, Fang, Yu Deng, Luying Yu, Ao Zhou, Jieting Wang, Jingyan Jia, Ning Li, Fadian Ding, Wei Lian, Qicai Liu, and et al. 2022. "A Macrophage Membrane–Polymer Hybrid Biomimetic Nanoplatform for Therapeutic Delivery of Somatostatin Peptide to Chronic Pancreatitis" Pharmaceutics 14, no. 11: 2341. https://doi.org/10.3390/pharmaceutics14112341
APA StyleWang, F., Deng, Y., Yu, L., Zhou, A., Wang, J., Jia, J., Li, N., Ding, F., Lian, W., Liu, Q., Yang, Y., & Lin, X. (2022). A Macrophage Membrane–Polymer Hybrid Biomimetic Nanoplatform for Therapeutic Delivery of Somatostatin Peptide to Chronic Pancreatitis. Pharmaceutics, 14(11), 2341. https://doi.org/10.3390/pharmaceutics14112341