Anti-Tumor Effects of Engineered VNP20009-Abvec-Igκ-mPD-1 Strain in Melanoma Mice via Combining the Oncolytic Therapy and Immunotherapy
Abstract
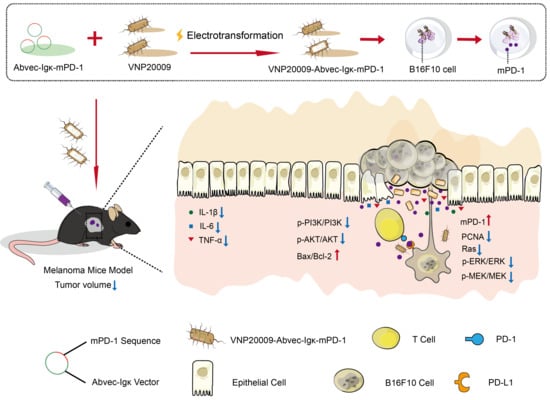
:1. Introduction
2. Materials and Methods
2.1. Construction of Engineered Strain and Evaluation In Vitro
2.2. The Ability of V-A-mPD-1 to Enter B16F10 and Express PD-1
2.3. Development and Treatment of Melanoma Mice Model
2.4. Pathological Histology
2.5. Real-Time Fluorescence Quantitative PCR
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Evaluation of V-A-mPD-1 In Vitro
3.2. V-A-mPD-1 Administration Inhibits Melanoma Growth in Mice
3.3. V-A-mPD-1 Inhibits Melanoma Growth by Suppressing Tumor Proliferation
3.4. V-A-mPD-1 Promotes Tumor Necrosis and Reduces Systemic Inflammatory Responses in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Qu, X.L.; Chen, Y. Identification of the potential prognostic genes of human melanoma. J. Cell. Physiol. 2019, 234, 9810–9815. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Scatena, C.; Murtas, D.; Tomei, S. Cutaneous Melanoma Classification: The Importance of High-Throughput Genomic Technologies. Front. Oncol. 2021, 11, 635488. [Google Scholar] [CrossRef] [PubMed]
- Redman, J.M.; Gibney, G.T.; Atkins, M.B. Advances in immunotherapy for melanoma. BMC Med. 2016, 14, 20. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.; Liu, Q.; Li, P.; Han, Y.; Bian, X.P.; Tang, Y.B.; Kong, Q.K. Endostatin gene therapy delivered by attenuated Salmonella typhimurium in murine tumor models. Cancer Gene Ther. 2018, 25, 167–183. [Google Scholar] [CrossRef]
- Coley, W.B., II. Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891, 14, 199–220. [Google Scholar] [CrossRef]
- Yazawa, K.; Fujimori, M.; Amano, J.; Kano, Y.; Taniguchi, S. Bifidobacterium longum as a delivery system for cancer gene therapy: Selective localization and growth in hypoxic tumors. Cancer Gene Ther. 2000, 7, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Kasinskas, R.W.; Forbes, N.S. Salmonella typhimurium Specifically Chemotax and Proliferate in Heterogeneous Tumor Tissue in Vitro. Biotechnol. Bioeng. 2006, 94, 710–721. [Google Scholar] [CrossRef]
- Wood, L.M.; Pan, Z.K.; Guirnalda, P.; Tsai, P.; Seavey, M.; Paterson, Y. Targeting tumor vasculature with novel Listeria-based vaccines directed against CD105. Cancer Immunol. Immunother. 2011, 60, 931–942. [Google Scholar] [CrossRef] [Green Version]
- Canale, F.P.; Basso, C.; Antonini, G.; Perotti, M.; Li, N.; Sokolovska, A.; Neumann, J.; James, M.J.; Geiger, S.; Jin, W.J.; et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 2021, 598, 662. [Google Scholar] [CrossRef]
- Liang, K.; Liu, Q.; Li, P.; Luo, H.Y.; Wang, H.J.; Kong, Q.K. Genetically engineered Salmonella Typhimurium: Recent advances in cancer therapy. Cancer Lett. 2019, 448, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Clairmont, C.; Lee, K.C.; Pike, J.; Ittensohn, M.; Low, K.B.; Pawelek, J.; Bermudes, D.; Brecher, S.M.; Margitich, D.; Turnier, J.; et al. Biodistribution and Genetic Stability of the Novel Antitumor Agent VNP20009, a Genetically Modified Strain of Salmonella typhimurium. J. Infect. Dis. 2000, 181, 1996–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toso, J.F.; Gill, V.J.; Hwu, P.; Marincola, F.M.; Restifo, N.P.; Schwartzentruber, D.J.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Stock, F.; et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. 2002, 20, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.; Nguyen, V.H.; D’Alincourt, M.S.; Manuel, E.R.; Kaltcheva, T.; Tsai, W.M.; Blazar, B.R.; Diamond, D.J.; Melstrom, L.G. Salmonella-mediated therapy targeting indoleamine 2, 3-dioxygenase 1 (IDO) activates innate immunity and mitigates colorectal cancer growth. Cancer Gene Ther. 2020, 27, 235–245. [Google Scholar] [CrossRef]
- King, I.; Bermudes, D.; Lin, S.; Belcourt, M.; Pike, J.; Troy, K.; Le, T.; Ittensohn, M.; Mao, J.; Lang, W.S.; et al. Tumor-targeted Salmonella expressing cytosine deaminase as an anticancer agent. Hum. Gene Ther. 2002, 13, 1225–1233. [Google Scholar] [CrossRef] [Green Version]
- Friedlos, F.; Lehouritis, P.; Ogilvie, L.; Hedley, D.; Davies, L.; Bermudes, D.; King, I.; Martin, J.; Marais, R.; Springer, C.J. Attenuated Salmonella targets prodrug activating enzyme carboxypeptidase G2 to mouse melanoma and human breast and colon carcinomas for effective suicide gene therapy. Clin. Cancer Res. 2008, 14, 4259–4266. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.X.; Yang, B.Y.; Cheng, X.W.; Qiao, Y.T.; Tang, B.; Chen, G.; Wei, J.; Liu, X.F.; Cheng, W.; Du, P.; et al. Salmonella-mediated tumor-targeting TRAIL gene therapy significantly suppresses melanoma growth in mouse model. Cancer Sci. 2012, 103, 325–333. [Google Scholar] [CrossRef]
- Chen, T.T.; Zhao, X.X.; Ren, Y.M.; Wang, Y.Q.; Tang, X.Y.; Tian, P.Y.; Wang, H.; Xin, H.B. Triptolide modulates tumour-colonisation and anti-tumour effect of attenuated Salmonella encoding DNase I. Appl. Microbiol. Biotechnol. 2019, 103, 929–939. [Google Scholar] [CrossRef]
- Wang, H.; Chen, T.T.; Wan, L.X.; Lu, J.C.; Wei, H.; Deng, K.-Y.; Wei, J.; Xin, H.-B. Attenuated Salmonella engineered with an apoptosis-inducing factor (AIF) eukaryotic expressing system enhances its anti-tumor effect in melanoma in vitro and in vivo. Appl. Microbiol. Biotechnol. 2020, 104, 3517–3528. [Google Scholar] [CrossRef]
- Lin, H.; Kryczek, I.; Li, S.S.; Green, M.D.; Ali, A.; Hamasha, R.; Wei, S.; Vatan, L.; Szeliga, W.; Grove, S.; et al. Stanniocalcin 1 is a phagocytosis checkpoint driving tumor immune resistance. Cancer Cell 2021, 39, 480. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Zhang, C.; Liu, X.L.; He, Z.F.; Shan, B.; Zeng, Q.X.; Zhao, Q.W.; Zhu, H.Y.; Liao, H.W.; Cen, X.F.; et al. ARIH1 signaling promotes anti-tumor immunity by targeting PD-L1 for proteasomal degradation. Nat. Commun. 2021, 12, 2346. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lu, X.; Luo, G.S.; Xiang, H. Progress in PD-1/PD-L1 pathway inhibitors: From biomacromolecules to small molecules. Eur. J. Med. Chem. 2020, 186, 111876. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Yi, M.; Jiao, D.C.; Xu, H.X.; Liu, Q.; Zhao, W.H.; Han, X.W.; Wu, K.M. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol. Cancer 2018, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Pedoeem, A.; Azoulay-Alfaguter, I.; Strazza, M.; Silverman, G.J.; Mor, A. Programmed death-1 pathway in cancer and autoimmunity. Clin. Immunol. 2014, 153, 145–152. [Google Scholar] [CrossRef]
- Du, P.; Hu, T.; An, Z.l.; Li, P.F.; Liu, L.H. In vitro and in vivo synergistic efficacy of ceritinib combined with programmed cell death ligand-1 inhibitor in anaplastic lymphoma kinase-rearranged non-small-cell lung cancer. Cancer Sci. 2020, 111, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.J.; Yi, M.; Qin, S.; Chu, Q.; Zheng, X.H.; Wu, K.M. The efficacy and safety of combination of PD-1 and CTLA-4 inhibitors: A meta-analysis. Exp. Hematol. Oncol. 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Su, L.J.; Morin, M.D.; Jones, B.T.; Mifune, Y.; Shi, H.X.; Wang, K.W.; Zhan, X.M.; Liu, A.J.; Wang, J.H.; et al. Adjuvant effect of the novel TLR1/TLR2 agonist Diprovocim synergizes with anti-PD-L1 to eliminate melanoma in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E8698–E8706. [Google Scholar] [CrossRef] [Green Version]
- Sato-Kaneko, F.; Yao, S.Y.; Ahmadi, A.; Zhang, S.S.; Hosoya, T.; Kaneda, M.M.; Varner, J.A.; Pu, M.; Messer, K.S.; Guiducci, C.; et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight 2017, 2, e93397. [Google Scholar] [CrossRef]
- Wang, G.; Kang, X.; Chen, K.S.; Jehng, T.; Jones, L.; Chen, J.; Huang, X.F.; Chen, S.-Y. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses. Nat. Commun. 2020, 11, 1395. [Google Scholar] [CrossRef]
- Wang, W.G.; Cheng, Y.H.; Yu, P.; Wang, H.R.; Zhang, Y.; Xu, H.H.; Ye, Q.S.; Yuan, A.H.; Hu, Y.Q.; Wu, J.H. Perfluorocarbon regulates the intratumoural environment to enhance hypoxia-based agent efficacy. Nat. Commun. 2019, 10, 1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, C.W.; Chen, Y.; Li, H.Y.; Li, W.Y.; Wei, J.; Li, Z.Y.; Wang, X.L.; Chen, T.T.; Huang, H. Engineered Bacteria EcN-MT Alleviate Liver Injury in Cadmium-Exposed Mice via its Probiotics Characteristics and Expressing of Metallothionein. Front. Pharmacol. 2022, 13, 857869. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.A. On detecting interference from endogenous antibodies in immunoassays by doubling dilutions test. Clin. Chem. Lab. Med. 2007, 45, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Chen, J.X.; Tang, B.; Zhang, X.Y.; Hua, Z.-C. Systemic administration of attenuated Salmonella typhimurium in combination with interleukin-21 for cancer therapy. Mol. Clin. Oncol. 2013, 1, 461–465. [Google Scholar] [CrossRef] [Green Version]
- Schneider, A.; Zhang, Y.H.; Zhang, M.Z.; Lu, W.J.; Rao, R.; Fan, X.F.; Redha, R.; Davis, L.; Breyer, R.M.; Harris, R.; et al. Membrane-associated PGE synthase-1 (mPGES-1) is coexpressed with both COX-1 and COX-2 in the kidney. Kidney Int. 2004, 65, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-H. Engineering bacteria toward tumor targeting for cancer treatment: Current state and perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 517–523. [Google Scholar] [CrossRef]
- Yu, B.; Yang, M.; Shi, L.; Yao, Y.D.; Jiang, Q.Q.; Li, X.F.; Tang, L.-H.; Zheng, B.-J.; Yuen, K.-Y.; Smith, D.K.; et al. Explicit hypoxia targeting with tumor suppression by creating an “obligate” anaerobic Salmonella typhimurium strain. Sci. Rep. 2012, 2, 436. [Google Scholar] [CrossRef] [Green Version]
- Thamm, D.H.; Kurzman, I.D.; King, I.; Li, Z.J.; Sznol, M.; Dubielzig, R.R.; Vail, D.M.; MacEwen, E.G. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: Phase I evaluation. Clin. Cancer Res. 2005, 11, 4827–4834. [Google Scholar] [CrossRef] [Green Version]
- Novotny, J.F.; Cogswell, J.; Inzunza, H.; Harbison, C.; Horak, C.; Averbuch, S. Establishing a complementary diagnostic for anti-PD-1 immune checkpoint inhibitor therapy. Ann. Oncol. 2016, 27, 1966–1969. [Google Scholar] [CrossRef]
- Wei, M.Q.; Ellem, K.A.O.; Dunn, P.; West, M.J.; Bai, C.X.; Vogelstein, B. Facultative or obligate anaerobic bacteria have the potential for multimodality therapy of solid tumours. Eur. J. Cancer 2007, 43, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Ko, I.O.; Jung, K.-H.; Kim, M.H.; Kang, K.J.; Lee, K.C.; Kim, K.M.; Noh, I.; Lee, Y.J.; Lim, S.M.; Kim, J.Y.; et al. Preliminary19F-MRS Study of Tumor Cell Proliferation with 3′-deoxy-3′-fluorothymidine and Its Metabolite (FLT-MP). Contrast Media Mol. Imaging 2017, 2017, 3981358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurikova, M.; Danihel, L.; Polak, S.; Varga, I. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Chen, Y.; Liu, X.Q.; Min, J.-J.; Tan, W.Z.; Zheng, J.H. Targeted cancer immunotherapy with genetically engineered oncolytic Salmonella typhimurium. Cancer Lett. 2020, 469, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Palrasu, M.; Knapinska, A.M.; Diez, J.; Smith, L.; LaVoi, T.; Giulianotti, M.; Houghten, R.A.; Fields, G.B.; Minond, D. A Novel Probe for Spliceosomal Proteins that Induces Autophagy and Death of Melanoma Cells Reveals New Targets for Melanoma Drug Discovery. Cell. Physiol. Biochem. 2019, 53, 656–686. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Liu, W.J.; Zhao, H.; Li, S.Z.; Guan, X.; Ying, J.M.; Zhang, Y.F.; Miao, F.; Zhang, M.M.; Ren, X.X.; et al. Rhomboid domain containing 1 promotes colorectal cancer growth through activation of the EGFR signalling pathway. Nat. Commun. 2015, 6, 8022. [Google Scholar] [CrossRef] [Green Version]
- Batista, A.; Rodvold, J.J.; Xian, S.; Searles, S.C.; Lew, A.; Iwawaki, T.; Almanza, G.; Waller, T.C.; Lin, J.; Jepsen, K.; et al. IRE1 alpha regulates macrophage polarization, PD-L1 expression, and tumor survival. PLoS Biol. 2020, 18, e3000687. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, X.P.; Ding, S.; Geng, Y.; Liu, J.Y.; Zhao, Z.M.; Zhang, R.; Xiao, X.; Wang, J.Y. A graph-based algorithm for estimating clonal haplotypes of tumor sample from sequencing data. BMC Med. Genom. 2019, 12, 27. [Google Scholar] [CrossRef] [Green Version]
- Chitrala, K.N.; Yang, X.M.; Busbee, B.; Singh, N.P.; Bonati, L.; Xing, Y.N.; Nagarkatti, P.; Nagarkatti, M. Computational prediction and in vitro validation of VEGFR1 as a novel protein target for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Sci. Rep. 2019, 9, 6810. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.J.; Niu, T.; Xiao, W. Uev1A promotes breast cancer cell survival and chemoresistance through the AKT-FOXO1-BIM pathway. Cancer Cell Int. 2019, 19, 331. [Google Scholar] [CrossRef]
- Itoh, N.; Semba, S.; Ito, M.; Takeda, H.; Kawata, S.; Yamakawa, M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer 2002, 94, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xia, Y.J.; Li, J.J.; Li, S.N.; Feng, J.; Wu, L.W.; Zhang, R.; Xu, S.Z.; Cheng, K.R.; Zhou, Y.Q.; et al. Shikonin Attenuates Concanavalin A-Induced Acute Liver Injury in Mice via Inhibition of the JNK Pathway. Mediat. Inflamm. 2016, 2016, 2748367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuruta, F.; Masuyama, N.; Gotoh, Y. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J. Biol. Chem. 2002, 277, 14040–14047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.-H.; Hu, W.; Zhang, X.-B.; Wang, W.; Zhang, L.-J. Protective Effect of Adrenomedullin on Rat Leydig Cells from Lipopolysaccharide-Induced Inflammation and Apoptosis via the PI3K/Akt Signaling Pathway ADM on Rat Leydig Cells from Inflammation and Apoptosis. Mediat. Inflamm. 2016, 2016, 7201549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peddareddigari, V.G.; Wang, D.Z.; Dubois, R.N. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. Off. J. Int. Cancer Microenviron. Soc. 2010, 3, 149–166. [Google Scholar] [CrossRef] [Green Version]
- Jedinak, A.; Dudhgaonkar, S.; Sliva, D. Activated macrophages induce metastatic behavior of colon cancer cells. Immunobiology 2010, 215, 242–249. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.-X.; Wang, X.-H.; Xu, X.; Chen, W.-J.; Wei, J.; Chen, T.-T.; Wei, H. Anti-Tumor Effects of Engineered VNP20009-Abvec-Igκ-mPD-1 Strain in Melanoma Mice via Combining the Oncolytic Therapy and Immunotherapy. Pharmaceutics 2022, 14, 2789. https://doi.org/10.3390/pharmaceutics14122789
Zhou D-X, Wang X-H, Xu X, Chen W-J, Wei J, Chen T-T, Wei H. Anti-Tumor Effects of Engineered VNP20009-Abvec-Igκ-mPD-1 Strain in Melanoma Mice via Combining the Oncolytic Therapy and Immunotherapy. Pharmaceutics. 2022; 14(12):2789. https://doi.org/10.3390/pharmaceutics14122789
Chicago/Turabian StyleZhou, De-Xi, Xiao-He Wang, Xuan Xu, Wen-Jie Chen, Jing Wei, Ting-Tao Chen, and Hong Wei. 2022. "Anti-Tumor Effects of Engineered VNP20009-Abvec-Igκ-mPD-1 Strain in Melanoma Mice via Combining the Oncolytic Therapy and Immunotherapy" Pharmaceutics 14, no. 12: 2789. https://doi.org/10.3390/pharmaceutics14122789
APA StyleZhou, D. -X., Wang, X. -H., Xu, X., Chen, W. -J., Wei, J., Chen, T. -T., & Wei, H. (2022). Anti-Tumor Effects of Engineered VNP20009-Abvec-Igκ-mPD-1 Strain in Melanoma Mice via Combining the Oncolytic Therapy and Immunotherapy. Pharmaceutics, 14(12), 2789. https://doi.org/10.3390/pharmaceutics14122789