Simultaneous Delivery of Econazole, Terbinafine and Amorolfine with Improved Cutaneous Bioavailability: A Novel Micelle-Based Antifungal “Tri-Therapy”
Abstract
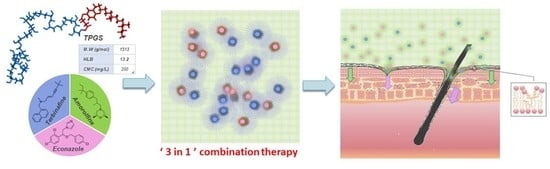
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
2.2.1. Quantification of ECZ, TBF and AMF by HPLC-UV
2.2.2. Quantification of ECZ, TBF and AMF by UHPLC-MS/MS
2.3. Development of TPGS Micelle-Based Antifungal “Tri-Therapy”
2.3.1. Preparation of Single Drug-Loaded Micelle Formulation
2.3.2. Characterization of Single Drug-Loaded Micelle Formulation
2.3.3. Preparation of Triple Antifungal Drug-Loaded Micelle Formulation
2.4. Skin Preparation
2.5. Evaluation of Cutaneous Delivery In Vitro
2.6. Investigation of Antifungal Biodistribution Profile
2.7. Antifungal Delivery to the PSU
2.8. Data Analysis
3. Results and Discussion
3.1. Characterization and Properties of Single Antifungal Drug-Loaded Micelles
3.2. Triple Antifungal Drug-Loaded Micelle Formulation
3.3. Cutaneous Delivery of the Antifungal Drugs
3.3.1. Antifungal Delivery under Infinite Dose
3.3.2. Antifungal Delivery under Finite Dose
3.4. Targeted Delivery to the PSU
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Schwartz, A.R. Superficial fungal infections. Lancet 2004, 364, 1173–1182. [Google Scholar] [CrossRef]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Grumbt, M.; Monod, M.; Yamada, T.; Hertweck, C.; Kunert, J.; Staib, P. Keratin Degradation by Dermatophytes Relies on Cysteine Dioxygenase and a Sulfite Efflux Pump. J. Investig. Dermatol. 2013, 133, 1550–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hube, B.; Hay, R.; Brasch, J.; Veraldi, S.; Schaller, M. Dermatomycoses and inflammation: The adaptive balance between growth, damage, and survival. J. Med. Mycol. 2015, 25, e44–e58. [Google Scholar] [CrossRef]
- Nenoff, P.; Krüger, C.; Ginter-Hanselmayer, G.; Tietz, H.-J. Mycology–An update. Part 1: Dermatomycoses: Causative agents, epidemiology and pathogenesis. JDDG J. Dtsch. Dermatol. Ges. 2014, 12, 188–210. [Google Scholar] [CrossRef]
- Marques, S.A.; Robles, A.M.; Tortorano, A.M.; Tuculet, M.A.; Negroni, R.; Mendes, R.P. Mycoses associated with AIDS in the Third World. Med Mycol. 2000, 38, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Keyser, D.P.; Backer, D.M.; Massart, D.L.; Westelinck, K. Two-week oral treatment of tinea pedis, comparing terbinafine (250 mg/day) with itraconazole (100 mg/day): A double-blind, multicentre study. Br. J. Dermatol. 1994, 130, 22–25. [Google Scholar] [CrossRef]
- Bell-Syer, S.E.M.; Khan, S.M.; Torgerson, D. Oral treatments for fungal infections of the skin of the foot. Cochrane Database Syst. Rev. 2012, 10, 003584. [Google Scholar] [CrossRef]
- Vandeputte, P.; Ferrari, S.; Coste, A.T. Antifungal Resistance and New Strategies to Control Fungal Infections. Int. J. Microbiol. 2012, 2012, 713687. [Google Scholar] [CrossRef]
- Heel, R.C.; Brogden, R.N.; Speight, T.M.; Avery, G.S. Econazole. Drugs 1978, 16, 177–201. [Google Scholar] [CrossRef]
- Daftardar, S.; Bahl, D.; Boddu, S.H.; Altorok, N.; Kahaleh, B. Ultrasound-mediated topical delivery of econazole nitrate with potential for treating Raynaud’s phenomenon. Int. J. Pharm. 2020, 580, 119229. [Google Scholar] [CrossRef] [PubMed]
- Firooz, A.; Nafisi, S.; Maibach, H.I. Novel drug delivery strategies for improving econazole antifungal action. Int. J. Pharm. 2015, 495, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Pathak, K. Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Bachhav, Y.; Mondon, K.; Kalia, Y.; Gurny, R.; Möller, M. Novel micelle formulations to increase cutaneous bioavailability of azole antifungals. J. Control. Release 2011, 153, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Nenoff, P.; Verma, S.B.; Vasani, R.; Burmester, A.; Hipler, U.; Wittig, F.; Krüger, C.; Nenoff, K.; Wiegand, C.; Saraswat, A.; et al. The current Indian epidemic of superficial dermatophytosis due toTrichophyton mentagrophytes—A molecular study. Mycoses 2018, 62, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.; Monod, M.; Salamin, K.; Burmester, A.; Uhrlaß, S.; Wiegand, C.; Hipler, U.-C.; Krüger, C.; Koch, D.; Wittig, F.; et al. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: A multicentre study. Mycoses 2020, 63, 717–728. [Google Scholar] [CrossRef]
- Evans, E.G. The rationale for combination therapy. Br. J. Dermatol. 2001, 145 (Suppl. 60), 9–13. [Google Scholar] [CrossRef]
- Polak, A. Combination of amorolfine with various antifungal drugs in dermatophytosis. Mycoses 2009, 36, 43–49. [Google Scholar] [CrossRef]
- Baudraz-Rosselet, F.; Ruffieux, C.; Lurati, M.; Bontems, O.; Monod, M. Onychomycosis Insensitive to Systemic Terbinafine and Azole Treatments Reveals Non-Dermatophyte Moulds as Infectious Agents. Dermatology 2010, 220, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Baran, R.; Kaoukhov, A. Topical antifungal drugs for the treatment of onychomycosis: An overview of current strategies for monotherapy and combination therapy. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 21–29. [Google Scholar] [CrossRef]
- Laurent, A. Antifungal Susceptibility Testing and Novel Pharmaco-Therapeutic Options for Topical Management of Onychomycoses; M Pharm Research Project; School of Pharmaceutical Sciences, University of Geneva: Geneva, Switzerland, 2016. [Google Scholar]
- Laurent, A.; Monod, M. Production ofTrichophyton rubrummicrospores in large quantities and its application to evaluate amorolfine/azole compound interactions in vitro. Mycoses 2017, 60, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Neubert, R.H.H.; Gensbügel, C.; Jäckel, A.; Wartewig, S. Different physicochemical properties of antimycotic agents are relevant for penetration into and through human nails. Die Pharm. 2006, 61, 604–607. [Google Scholar]
- Zhang, Z.; Tan, S.; Feng, S.-S. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials 2012, 33, 4889–4906. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Zhu, Z.; Fang, D.; Sun, Y.; Kang, C.; Di, L.; Zhang, L.; Gao, Y. Redox-triggered mitoxantrone prodrug micelles for overcoming multidrug-resistant breast cancer. J. Drug Target. 2017, 26, 75–85. [Google Scholar] [CrossRef]
- Jin, Q.; Li, H.; Jin, Z.; Huang, L.; Wang, F.; Zhou, Y.; Liu, Y.; Jiang, C.; Oswald, J.; Wu, J.; et al. TPGS modified nanoliposomes as an effective ocular delivery system to treat glaucoma. Int. J. Pharm. 2018, 553, 21–28. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Chen, X.; Su, J.; Huang, C. Enhanced efficacy of baicalin-loaded TPGS polymeric micelles against periodontitis. Mater. Sci. Eng. C 2019, 101, 387–395. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Pandey, M.; Kumbhar, S.A.; Tekade, R.K.; Iyer, A.K.; Kesharwani, P. Recent advances in TPGS-based nanoparticles of docetaxel for improved chemotherapy. Int. J. Pharm. 2017, 529, 506–522. [Google Scholar] [CrossRef]
- Collnot, E.-M.; Baldes, C.; Schaefer, U.F.; Edgar, K.J.; Wempe, M.F.; Lehr, C.-M. Vitamin E TPGS P-Glycoprotein Inhibition Mechanism: Influence on Conformational Flexibility, Intracellular ATP Levels, and Role of Time and Site of Access. Mol. Pharm. 2010, 7, 642–651. [Google Scholar] [CrossRef]
- Constantinides, P.P.; Han, J.; Davis, S.S. Advances in the Use of Tocols as Drug Delivery Vehicles. Pharm. Res. 2006, 23, 243–255. [Google Scholar] [CrossRef]
- Baert, L.; van’t Klooster, G.; Dries, W.; François, M.; Wouters, A.; Basstanie, E.; Iterbeke, K.; Stappers, F.; Stevens, P.; Schueller, L.; et al. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur. J. Pharm. Biopharm. 2009, 72, 502–508. [Google Scholar] [CrossRef]
- Kandekar, S.G.; del Río-Sancho, S.; Lapteva, M.; Kalia, Y.N. Selective delivery of adapalene to the human hair follicle under finite dose conditions using polymeric micelle nanocarriers. Nanoscale 2018, 10, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Quartier, J.; Lapteva, M.; Boulaguiem, Y.; Guerrier, S.; Kalia, Y.N. Polymeric micelle formulations for the cutaneous delivery of sirolimus: A new approach for the treatment of facial angiofibromas in tuberous sclerosis complex. Int. J. Pharm. 2021, 604, 120736. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, M.; Mondon, K.; Möller, M.; Gurny, R.; Kalia, Y.N. Polymeric Micelle Nanocarriers for the Cutaneous Delivery of Tacrolimus: A Targeted Approach for the Treatment of Psoriasis. Mol. Pharm. 2014, 11, 2989–3001. [Google Scholar] [CrossRef]
- Lapteva, M.; Möller, M.; Gurny, R.; Kalia, Y.N. Self-assembled polymeric nanocarriers for the targeted delivery of retinoic acid to the hair follicle. Nanoscale 2015, 7, 18651–18662. [Google Scholar] [CrossRef] [Green Version]
- Danafar, H.; Jaberizadeh, H.; Andalib, S. In vitro and in vivo delivery of gliclazide loaded mPEG-PCL micelles and its kinetic release and solubility study. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pöppler, A.; Lübtow, M.M.; Schlauersbach, J.; Wiest, J.; Meinel, L.; Luxenhofer, R. Loading-Dependent Structural Model of Polymeric Micelles Encapsulating Curcumin by Solid-State NMR Spectroscopy. Angew. Chem. Int. Ed. 2019, 58, 18540–18546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondon, K.D. Novel Micellar Systems for the Formulation of Poorly Water Soluble Drugs: Biocompatibility Aspects and Pharmaceutical Applications. Ph.D. Thesis, Université de Genève, Geneva, Switzerland, 2010. [Google Scholar]
- Naeff, R. Feasibility of topical liposome drugs produced on an industrial scale. Adv. Drug Deliv. Rev. 1996, 18, 343–347. [Google Scholar] [CrossRef]
- Yu, L.; Bridgers, A.; Polli, J.; Vickers, A.; Long, S.; Roy, A.; Winnike, R.; Coffin, M. Vitamin E-TPGS increases absorption flux of an HIV protease inhibitor by enhancing its solubility and permeability. Pharm. Res. 1999, 16, 1812–1817. [Google Scholar] [CrossRef]
- Ghosh, I.; Michniak-Kohn, B. A comparative study of Vitamin E TPGS/HPMC supersaturated system and other solubilizer/polymer combinations to enhance the permeability of a poorly soluble drug through the skin. Drug Dev. Ind. Pharm. 2012, 38, 1408–1416. [Google Scholar] [CrossRef]
- Quartier, J.; Rao, W.; Slade, S.; Métral, F.; Lapteva, M.; Kalia, Y.N. DESI-MS imaging to visualize spatial distribution of xenobiotics and endogenous lipids in the skin. Int. J. Pharm. 2021, 607, 120967. [Google Scholar] [CrossRef]
- Trauer, S.; Lademann, J.; Knorr, F.; Richter, H.; Liebsch, M.; Rozycki, C.; Balizs, G.; Büttemeyer, R.; Linscheid, M.; Patzelt, A. Development of an in vitro Modified Skin Absorption Test for the Investigation of the Follicular Penetration Pathway of Caffeine. Ski. Pharmacol. Physiol. 2010, 23, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Otberg, N.; Patzelt, A.; Rasulev, U.; Hagemeister, T.; Linscheid, M.; Sinkgraven, R.; Sterry, W.; Lademann, J. The role of hair follicles in the percutaneous absorption of caffeine. Br. J. Clin. Pharmacol. 2007, 65, 488–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahmana, N.; Mugnier, T.; Gabriel, D.; Favez, T.; Kowalczuk, L.; Behar-Cohen, F.; Gurny, R.; Kalia, Y.N. Polymeric micelle mediated follicular delivery of spironolactone: Targeting the mineralocorticoid receptor to prevent glucocorticoid-induced activation and delayed cutaneous wound healing. Int. J. Pharm. 2021, 604, 120773. [Google Scholar] [CrossRef] [PubMed]







| # a | P.C. | D:P ratio | T.L. | ECZ | TBF | ||||
|---|---|---|---|---|---|---|---|---|---|
| D.L. | I.E. | D.C. | D.L. | I.E. | D.C. | ||||
| 1 | 25 | 1:20 | 50 | 55.96 ± 0.33 | 109.72 ± 0.64 | 1.40 ± 0.01 | 52.78 ± 0.85 | 103.48 ± 1.67 | 1.32 ± 0.02 |
| 2 | 25 | 1:10 | 100 | 104.18 ± 0.42 | 103.14 ± 0.42 | 2.60 ± 0.01 | 94.85 ± 0.59 | 92.53 ± 0.58 | 2.37 ± 0.01 |
| 3 | 25 | 1:5 | 200 | 207.74 ± 0.92 | 102.33 ± 0.45 | 5.19 ± 0.02 | 216.21 ± 6.58 | 107.57 ± 3.28 | 5.41 ± 0.16 |
| 4 | 25 | 2:5 | 400 | 151.43 ± 1.71 | 36.93 ± 0.42 | 3.79 ± 0.04 | 380.00 ±17.24 | 94.76 ± 4.30 | 9.50 ± 0.43 |
| #a | ECZ | TBF | ||||||
|---|---|---|---|---|---|---|---|---|
| Zav | P.I. | dn | dv | Zav | P.I. | dn | dv | |
| 1 | 4.982 | 0.184 | 3.315 | 3.908 | 7.084 | 0.051 | 5.257 | 6.162 |
| 2 | 4.675 | 0.122 | 3.148 | 3.862 | 7.114 | 0.057 | 5.274 | 6.189 |
| 3 | 8.105 | 0.061 | 5.927 | 7.018 | 8.561 | 0.195 | 6.204 | 7.319 |
| 4 | 5.118 | 0.145 | 3.175 | 4.019 | 9.116 | 0.062 | 6.738 | 7.937 |
| F | ECZ | TBF | ||||||
|---|---|---|---|---|---|---|---|---|
| D.L. | I.E. | D.C. | P.C. | D.L. | I.E. | D.C. | P.C. | |
| A | 104.18 ± 0.42 | 103.14 ± 0.42 | 2.60 ± 0.01 | 25 | 94.85 ± 0.59 | 92.53 ± 0.58 | 2.37 ± 0.01 | 25 |
| B | 104.18 ± 0.42 | 103.14 ± 0.42 | 1.01 ± 0.01 | 9.6 | 94.85 ± 0.59 | 92.53 ± 0.58 | 1.08 ± 0.02 | 10.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gou, S.; Monod, M.; Salomon, D.; Kalia, Y.N. Simultaneous Delivery of Econazole, Terbinafine and Amorolfine with Improved Cutaneous Bioavailability: A Novel Micelle-Based Antifungal “Tri-Therapy”. Pharmaceutics 2022, 14, 271. https://doi.org/10.3390/pharmaceutics14020271
Gou S, Monod M, Salomon D, Kalia YN. Simultaneous Delivery of Econazole, Terbinafine and Amorolfine with Improved Cutaneous Bioavailability: A Novel Micelle-Based Antifungal “Tri-Therapy”. Pharmaceutics. 2022; 14(2):271. https://doi.org/10.3390/pharmaceutics14020271
Chicago/Turabian StyleGou, Si, Michel Monod, Denis Salomon, and Yogeshvar N. Kalia. 2022. "Simultaneous Delivery of Econazole, Terbinafine and Amorolfine with Improved Cutaneous Bioavailability: A Novel Micelle-Based Antifungal “Tri-Therapy”" Pharmaceutics 14, no. 2: 271. https://doi.org/10.3390/pharmaceutics14020271
APA StyleGou, S., Monod, M., Salomon, D., & Kalia, Y. N. (2022). Simultaneous Delivery of Econazole, Terbinafine and Amorolfine with Improved Cutaneous Bioavailability: A Novel Micelle-Based Antifungal “Tri-Therapy”. Pharmaceutics, 14(2), 271. https://doi.org/10.3390/pharmaceutics14020271