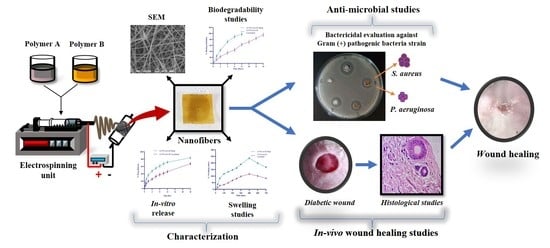
Multifunctional Biomimetic Nanofibrous Scaffold Loaded with Asiaticoside for Rapid Diabetic Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrospinning Solutions
2.3. Fabrication of AT Loaded Electrospun Nanofibers
2.4. Crosslinking of Nanofibers
2.5. Surface Morphology of AT Loaded Electrospun Nanofibers
2.6. Mechanical Strength of Nanofibers
2.7. Measurement of Swelling Ratio or Water Uptake
2.8. Measurement of In Vitro Biodegradability
2.9. In Vitro Drug Release Study
2.10. Anti-Microbial Activity
2.10.1. Formation of the Zone of Inhibition
2.10.2. Time-Kill Assay
2.10.3. Microbial Penetration Test
2.11. In Vitro Cell-Line Study
2.11.1. Cell Culture and Treatment
2.11.2. In Vitro Cytotoxicity (MTT Assay)
2.11.3. In Vitro Scratch (Cell Migration) Assay
2.12. In Vivo Studies
2.12.1. Inducing Diabetes and Measuring Body Weight
2.12.2. In Vivo Wound Healing Study
2.13. Histopathology
2.14. Statistical Analysis
3. Results and Discussion
3.1. Morphology of AT-Loaded Nanofibers
3.2. Mechanical Strength of Nanofibers
3.3. Swelling Ratio or Water Uptake Study
3.4. In-Vitro Biodegradation Study
3.5. In Vitro Drug Release
3.6. Anti-Microbial Study
3.6.1. Formation of Zone of Inhibition
3.6.2. Time Kill Assay
3.6.3. Microbial Penetration
3.7. In Vitro Cytotoxicity (MTT Assay)
3.8. In Vitro Scratch Assay
3.9. Measurement of Body Weight and Diabetes
3.10. In Vivo Wound Healing Study
3.11. Histopathology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar]
- Moura, L.I.; Dias, A.M.; Carvalho, E.; de Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samadian, H.; Zamiri, S.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Khastar, H.; Alam, M.; Ai, A.; Derakhshankhah, H.; Allahyari, Z. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In vitro and in vivo studies. Sci. Rep. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Rodgers, K.; Verco, S.; Bolton, L.; Dizerega, G. Accelerated healing of diabetic wounds by NorLeu3-angiotensin (1–7). Expert Opin. Investig. Drugs 2011, 20, 1575–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Gupta, A.; Upadhyay, N.K.; Sawhney, R.; Kumar, R. A poly-herbal formulation accelerates normal and impaired diabetic wound healing. Wound Repair Regen. 2008, 16, 784–790. [Google Scholar] [CrossRef]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Designing electrospun nanofiber mats to promote wound healing—A review. J. Mater. Chem. A 2013, 1, 4531–4541. [Google Scholar] [CrossRef] [Green Version]
- Shukla, A.; Rasik, A.; Jain, G.; Shankar, R.; Kulshrestha, D.; Dhawan, B.J. In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J. Ethnopharmacol. 1999, 65, 1–11. [Google Scholar] [CrossRef]
- Maquart, F.-X.; Bellon, G.; Gillery, P.; Wegrowski, Y.; Borel, J.-P. Stimulation of collagen synthesis in fibroblast cultures by a triterpene extracted from Centella asiatica. Connect. Tissue Res. 1990, 24, 107–120. [Google Scholar] [CrossRef]
- Shukla, A.; Rasik, A.M.; Patnaik, G.K. Depletion of reduced glutathione, ascorbic acid, vitamin E and antioxidant defence enzymes in a healing cutaneous wound. Free Radic. Res. 1997, 26, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Gizaw, M.; Thompson, J.; Faglie, A.; Lee, S.-Y.; Neuenschwander, P.; Chou, S.-F. Electrospun fibers as a dressing material for drug and biological agent delivery in wound healing applications. Bioengineering 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Zhang, Y.; Zhang, D.; Tang, Z.; Chen, X.; Wang, C. Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int. J. Biol. Macromol. 2014, 68, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Zhang, H.; Shi, X.; Zhao, J.; Chen, Y.; Wu, F.; Yang, J.; Li, X. Asiaticoside nitric oxide gel accelerates diabetic cutaneous ulcers healing by activating Wnt/β-catenin signaling pathway. Int. Immunopharmacol. 2020, 79, 106109. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Du, L.; Jin, Y. Pharmacotherapy. Preparation of asiaticoside-loaded coaxially electrospinning nanofibers and their effect on deep partial-thickness burn injury. Biomed. Pharmacother. 2016, 83, 33–40. [Google Scholar] [CrossRef]
- Agarwal, Y.; Rajinikanth, P.; Ranjan, S.; Tiwari, U.; Balasubramnaiam, J.; Pandey, P.; Arya, D.K.; Anand, S.; Deepak, P. Curcumin loaded polycaprolactone-/polyvinyl alcohol-silk fibroin based electrospun nanofibrous mat for rapid healing of diabetic wound: An in-vitro and in-vivo studies. Int. J. Biol. Macromol. 2021, 176, 376–386. [Google Scholar] [CrossRef]
- Sasithorn, N.; Martinová, L. Fabrication of silk nanofibres with needle and roller electrospinning methods. J. Nanomater. 2014, 140. [Google Scholar] [CrossRef]
- Širc, J.; Hobzova, R.; Kostina, N.; Munzarová, M.; Juklíčková, M.; Lhotka, M.; Kubinová, Š.; Zajícová, A.; Michálek, J. Morphological characterization of nanofibers: Methods and application in practice. J. Nanomater. 2012, 121. [Google Scholar] [CrossRef]
- Tan, E.; Lim, C.T. Mechanical characterization of nanofibers—A review. Compos. Sci. Technol. 2006, 66, 1102–1111. [Google Scholar] [CrossRef]
- Yun, J.; Im, J.S.; Lee, Y.-S.; Kim, H.-I. Electro-responsive transdermal drug delivery behavior of PVA/PAA/MWCNT nanofibers. Eur. Polym. J. 2011, 47, 1893–1902. [Google Scholar] [CrossRef]
- Ye, P.; Wei, S.; Luo, C.; Wang, Q.; Li, A.; Wei, F. Long-term effect against methicillin-resistant staphylococcus aureus of emodin released from coaxial electrospinning nanofiber membranes with a biphasic profile. Biomolecules 2020, 10, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonseca, Á.; Menes, O.; Giménez, E. A comparative study of the mechanical, shape-memory, and degradation properties of poly (lactic acid) nanofiber and cellulose nanocrystal reinforced poly (mannitol sebacate) nanocomposites. RSC Adv. 2017, 7, 21869–21882. [Google Scholar] [CrossRef] [Green Version]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appiah, T.; Boakye, Y.D.; Agyare, C. Antimicrobial activities and time-kill kinetics of extracts of selected Ghanaian mushrooms. Evid. Based Complementary Altern. Med. 2017, 4534350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samadian, H.; Salehi, M.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Sahrapeyma, H.; Goodarzi, A.; Ghorbani, S. In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 964–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wsoo, M.A.; Abd Razak, S.I.; Bohari, S.P.M.; Shahir, S.; Salihu, R.; Kadir, M.R.A.; Nayan, N.H. Vitamin D3-loaded electrospun cellulose acetate/polycaprolactone nanofibers: Characterization, in-vitro drug release and cytotoxicity studies. Int. J. Biol. Macromol. 2021, 181, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Muniandy, K.; Gothai, S.; Tan, W.S.; Kumar, S.S.; Mohd Esa, N.; Chandramohan, G.; Al-Numair, K.S.; Arulselvan, P. In vitro wound healing potential of stem extract of Alternanthera sessilis. Evid. Based Complementary Altern. Med. 2018, 3142073. [Google Scholar] [CrossRef] [Green Version]
- Poornima, B.; Korrapati, P.S. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr. Polym. 2017, 157, 1741–1749. [Google Scholar] [CrossRef]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-Rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Dolatshahi-Pirouz, A.; Zhang, Y.S.; Kundu, S.C.; Akbari, M. Hyaluronic acid (HA)-based silk fibroin/zinc oxide core–shell electrospun dressing for burn wound management. Macromol. Biosci. 2020, 20, 1900328. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Norouzian, D.; Mehrabi, M.; Jamshidi, S.; Farhangi, A.; Verdi, A.A.; Mofidian, S.; Rad, B.L. Induction of diabetes by streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulbake, U.; Jain, S.; Kumar, N.; Mittal, A. Curcumin loaded biomimetic composite graft for faster regeneration of skin in diabetic wounds. J. Drug Deliv. Sci. Technol. 2018, 47, 12–21. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Antunes, J.C.; Amorim, M.T.P.; Felgueiras, H.P. Green Optimization of Glutaraldehyde Vapor-Based Crosslinking on Poly (Vinyl Alcohol)/Cellulose Acetate Electrospun Mats for Applications as Chronic Wound Dressings. Proceedings 2020, 69, 30. [Google Scholar] [CrossRef]
- Hulupi, M.; Haryadi, H. Synthesis and characterization of electrospinning PVA nanofiber-crosslinked by glutaraldehyde. Mater. Today 2019, 13, 199–204. [Google Scholar] [CrossRef]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine 2017, 12, 1335–1352. [Google Scholar] [CrossRef] [PubMed]
- Akhshabi, S.; Biazar, E.; Singh, V.; Heidari Keshel, S.; Nagaraja, G. The effect of glutaraldehyde cross-linker on structural and biocompatibility properties of collagen-chondroitin sulfate electrospun mat. Mater. Technol. 2018, 33, 253–261. [Google Scholar] [CrossRef]
- Tian, H.; Yuan, L.; Wang, J.; Wu, H.; Wang, H.; Xiang, A.; Ashok, B.; Rajulu, A.V. Electrospinning of polyvinyl alcohol into crosslinked nanofibers: An approach to fabricate functional adsorbent for heavy metals. J. Hazard. Mater. 2019, 378, 120751. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.M.; Gaudino, G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp. Cell Res. 2005, 304, 274–286. [Google Scholar] [CrossRef]
- Waghmare, V.S.; Wadke, P.R.; Dyawanapelly, S.; Deshpande, A.; Jain, R.; Dandekar, P. Starch based nanofibrous scaffolds for wound healing applications. Bioact. Mater. 2018, 3, 255–266. [Google Scholar] [CrossRef]















| S. No. | Formulations | Strength (MPa) |
|---|---|---|
| 1. | AT-SF-NF | 6.04 ± 0.56 |
| 2. | AT-PVA-SA-SF (Non-crosslinked) | 17.73 ± 1.23 |
| 3. | AT-PVA-SA-SF (Crosslinked) | 20.65 ± 1.79 |
| Groups | Mean Sugar Level (mg/dL) | ||
|---|---|---|---|
| 0 Day | 3rd Day | 14th Day | |
| Before Inducing Diabetes | After Inducing Diabetes | ||
| NC | 90 ± 4.54 | 170 ± 4.68 | 110 ± 2.63 |
| TC | 100 ± 4.28 | 300 ± 4.57 | 130 ± 4.38 |
| F1 | 95 ± 3.87 | 250 ± 3.65 | 120 ± 3.22 |
| F2 | 100 ± 4.99 | 280 ± 4.62 | 135 ± 4.51 |
| Groups | Mean Body Weight (gm) | ||
|---|---|---|---|
| 0 Day | 3rd Day | 14th Day | |
| Before Inducing Diabetes | After Inducing Diabetes | ||
| NC | 130 ± 2.08 | 100 ± 2.10 | 125 ± 2.19 |
| TC | 130 ± 2.17 | 105 ± 2.31 | 134 ± 2.67 |
| F1 | 145 ± 3.02 | 110 ± 3.08 | 140 ± 3.48 |
| F2 | 150 ± 2.98 | 130 ± 2.88 | 140 ± 2.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anand, S.; Rajinikanth, P.S.; Arya, D.K.; Pandey, P.; Gupta, R.K.; Sankhwar, R.; Chidambaram, K. Multifunctional Biomimetic Nanofibrous Scaffold Loaded with Asiaticoside for Rapid Diabetic Wound Healing. Pharmaceutics 2022, 14, 273. https://doi.org/10.3390/pharmaceutics14020273
Anand S, Rajinikanth PS, Arya DK, Pandey P, Gupta RK, Sankhwar R, Chidambaram K. Multifunctional Biomimetic Nanofibrous Scaffold Loaded with Asiaticoside for Rapid Diabetic Wound Healing. Pharmaceutics. 2022; 14(2):273. https://doi.org/10.3390/pharmaceutics14020273
Chicago/Turabian StyleAnand, Sneha, Paruvathanahalli Siddalingam Rajinikanth, Dilip Kumar Arya, Prashant Pandey, Ravi K. Gupta, Ruchi Sankhwar, and Kumarappan Chidambaram. 2022. "Multifunctional Biomimetic Nanofibrous Scaffold Loaded with Asiaticoside for Rapid Diabetic Wound Healing" Pharmaceutics 14, no. 2: 273. https://doi.org/10.3390/pharmaceutics14020273
APA StyleAnand, S., Rajinikanth, P. S., Arya, D. K., Pandey, P., Gupta, R. K., Sankhwar, R., & Chidambaram, K. (2022). Multifunctional Biomimetic Nanofibrous Scaffold Loaded with Asiaticoside for Rapid Diabetic Wound Healing. Pharmaceutics, 14(2), 273. https://doi.org/10.3390/pharmaceutics14020273