Controlled Release of Caffeic Acid and Pinocembrin by Use of nPSi-βCD Composites Improves Their Antiangiogenic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Synthesis of nPSi-βCD Composites
2.3. Physicochemical Characterization
2.4. Loading and Release of Polyphenols
2.5. Cell Culture
2.6. Cytotoxicity Assays
2.7. Oxidative Stress Induction
2.8. Evaluation of Antiangiogenic Activity of Caffeic Acid and Pinocembrin
2.8.1. Chick-CAM Assay
2.8.2. Formation of Tubular Structures in the Matrigel
2.9. Determination of the the Antioxidant Activity of Polyphenols
2.10. Evaluation of Gene Expression
2.10.1. RNA Extraction
2.10.2. Real Time PCR
2.11. Statistical Analysis
3. Results and Discussion
3.1. Hydrogen Peroxide Cytotoxicity
3.2. Oxidative Stress Induction
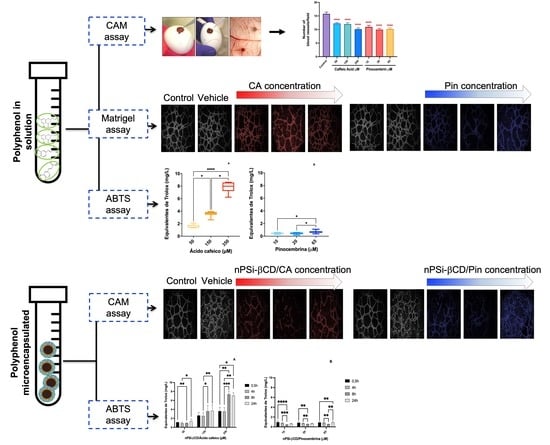
3.3. The Antiangiogenic Effect of the Treatment with Caffeic Acid and Pinocembrin in Solution on the Formation of Vessels in the Chorioallantoic Membrane of Embryonated Chicken Eggs
3.4. The Synthesis of Composite Microparticles nPSi-βCD and Physicochemical Characterization
3.5. The Effect of the Treatment with Caffeic Acid and Pinocembrin in Solution and Loaded in the nPSi-βCD Composite Microparticle on the Capacity of HUVECs to Form Tubular Structures in Matrigel
3.6. Antioxidant Capacity of Polyphenols in Solution
3.7. Kinetics of Antioxidant Capacity of Polyphenols Loaded in the nPSi-βCD Composite Microparticle
3.8. The Effect of the Treatment with Caffeic Acid and Pinocembrin in Solution and Loaded in the nPSi-βCD Composite Microparticle on the Expression of Genes Nrf2, Keap1, Akt, Cat, Glut-P, Hmox-1, Gclc, and Gclm Related to the Antioxidant Pathway
3.9. The Effect of the Treatment with Caffeic Acid and Pinocembrin in Soluion and Loaded in the nPSi-βCD Composite Microparticle on the Expression of Genes Nrf2, Keap1, Akt, Cat, Glut-P, Hmox1, Gclc, and Gclm Related to the Antioxidant Pathway
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Favier, J.; Corvol, P. Angiogénèse physiologique [Physiological angiogenesis]. Therapie 2001, 56, 455–463. [Google Scholar] [PubMed]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 385, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Heistad, D.D.; Marcus, M.L.; Larsen, G.E.; Armstrong, M.L. Role of vasa vasorum in nourishment of the aortic wall. Am. J. Physiol. 1981, 240, H781–H787. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, Z. Angiogenesis. BioMed Res. Int. 2015, 2015, 135861. [Google Scholar] [CrossRef]
- Krupinski, J.; Kaluza, J.; Kumar, P.; Kumar, S.; Wang, J.M. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994, 25, 1794–1798. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Alexopoulos, N.; Raggi, P. Calcification in atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 681–688. [Google Scholar] [CrossRef]
- Virmani, R.; Joner, M.; Sakakura, K. Recent highlights of ATVB: Calcification. Arter. Thromb. Vasc. Biol. 2014, 34, 1329–1332. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Pasterkamp, G. Requiem for the ‘vulnerable plaque’. Eur. Heart J. 2015, 36, 2984–2987. [Google Scholar] [CrossRef] [Green Version]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Contribution of neovascularization and intraplaque haemorrhage to atherosclerotic plaque progression and instability. Acta Physiol. 2015, 213, 539–553. [Google Scholar] [CrossRef]
- Tanaka, K.; Nagata, D.; Hirata, Y.; Tabata, Y.; Nagai, R.; Sata, M. Augmented angiogenesis in adventitia promotes growth of atherosclerotic plaque in apolipoprotein E-deficient mice. Atherosclerosis 2011, 215, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, P.; Emini Veseli, B.; Van der Veken, B.; Roth, L.; Martinet, W.; De Meyer, G.R.Y. Pharmacological strategies to inhibit intra-plaque angiogenesis in atherosclerosis. Vascul. Pharmacol. 2019, 112, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Sluimer, J.C.; Kolodgie, F.D.; Bijnens, A.P.; Maxfield, K.; Pacheco, E.; Kutys, B.; Duimel, H.; Frederik, P.M.; van Hinsbergh, V.W.; Virmani, R.; et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J. Am. Coll. Cardiol. 2009, 53, 1517–1527. [Google Scholar] [CrossRef] [Green Version]
- Moreno, P.R.; Purushothaman, K.R.; Fuster, V.; Echeverri, D.; Truszczynska, H.; Sharma, S.K.; Badimon, J.J.; O’Connor, W.N. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: Implications for plaque vulnerability. Circulation 2004, 110, 2031–2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Hou, J.; Xing, L.; Kim, S.J.; Yonetsu, T.; Kato, K.; Lee, H.; Zhang, S.; Yu, B.; Jang, I.K. Significance of intraplaque neovascularisation for vulnerability: Optical coherence tomography study. Heart 2012, 98, 1504–1509. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef]
- McMahon, M.; Lamont, D.J.; Beattie, K.A.; Hayes, J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. USA 2010, 107, 18838–18843. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Jang, H.D. Nrf2-mediated HO-1 induction coupled with the ERK signaling pathway contributes to indirect antioxidant capacity of caffeic acid phenethyl ester in HepG2 cells. Int. J. Mol. Sci. 2014, 15, 12149–12165. [Google Scholar] [CrossRef] [Green Version]
- Costa, T.J.; Barros, P.R.; Arce, C.; Santos, J.D.; da Silva-Neto, J.; Egea, G.; Dantas, A.P.; Tostes, R.C.; Jiménez-Altayó, F. The homeostatic role of hydrogen peroxide, superoxide anion and nitric oxide in the vasculature. Free Radic. Biol. Med. 2021, 162, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Motori, E.; Fabbri, D.; Malaguti, M.; Leoncini, E.; Lorenzini, A.; Hrelia, S. H2O2 preconditioning modulates phase II enzymes through p38 MAPK and PI3K/Akt activation. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2196–H2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, L.; Yang, C.; Gu, M.; Zheng, D.; Lin, L.; Wang, X.; Lan, A.; Hu, F.; Feng, J. PI3K/Akt signaling pathway-induced heme oxygenase-1 upregulation mediates the adaptive cytoprotection of hydrogen peroxide preconditioning against oxidative injury in PC12 cells. Int. J. Mol. Med. 2012, 30, 314–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z. Atherosclerosis and atheroma plaque rupture: Imaging modalities in the visualization of vasa vasorum and atherosclerotic plaques. Sci. World J. 2014, 2014, 312764. [Google Scholar] [CrossRef] [PubMed]
- Woodman, O.L.; Chan, E.C. Vascular and anti-oxidant actions of flavonols and flavones. Clin. Exp. Pharmacol. Physiol. 2004, 31, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, L.; Wojnar, W.; Zych, M.; Wyględowska-Promieńska, D.; Mrukwa-Kominek, E.; Kaczmarczyk-Sedlak, I. Effect of Resveratrol, a Dietary-Derived Polyphenol, on the Oxidative Stress and Polyol Pathway in the Lens of Rats with Streptozotocin-Induced Diabetes. Nutrients 2018, 10, 1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijak, M.; Ziewiecki, R.; Saluk, J.; Ponczek, M.; Pawlaczyk, I.; Krotkiewski, H.; Wachowicz, B.; Nowak, P. Thrombin inhibitory activity of some polyphenolic compounds. Med. Chem. Res. 2014, 23, 2324–2337. [Google Scholar] [CrossRef] [Green Version]
- Paeng, S.H.; Jung, W.K.; Park, W.S.; Lee, D.S.; Kim, G.Y.; Choi, Y.H.; Seo, S.K.; Jang, W.H.; Choi, J.S.; Lee, Y.M.; et al. Caffeic acid phenethyl ester reduces the secretion of vascular endothelial growth factor through the inhibition of the ROS, PI3K and HIF-1α signaling pathways in human retinal pigment epithelial cells under hypoxic conditions. Int. J. Mol. Med. 2015, 35, 1419–1426. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, F.; Fang, L.; Cai, R.; Zong, C.; Qi, Y. Genkwanin inhibits proinflammatory mediators mainly through the regulation of miR-101/MKP-1/MAPK pathway in LPS-activated macrophages. PLoS ONE 2014, 9, e96741. [Google Scholar] [CrossRef]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential Effects of Pomegranate Polyphenols in Cancer Prevention and Therapy. Oxid. Med. Cell Longev. 2015, 2015, 938475. [Google Scholar] [CrossRef] [Green Version]
- Diniz, C.; Suliburska, J.; Ferreira, I.M.P.L.V.O. New insights into the antiangiogenic and proangiogenic properties of dietary polyphenols. Mol. Nutr. Food Res. 2017, 61, 1600912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, X.B.; Zhao, J.C.; Gao, X.F.; Zhang, X.N.; Hou, K. Neuroprotective effect of resveratrol against radiation after surgically induced brain injury by reducing oxidative stress, inflammation, and apoptosis through NRf2/HO-1/NF-κB signaling pathway. J. Biochem. Mol. Toxicol. 2020, 34, e22600. [Google Scholar] [CrossRef] [PubMed]
- Pandareesh, M.D.; Mythri, R.B.; Srinivas Bharath, M.M. Bioavailability of dietary polyphenols: Factors contributing to their clinical application in CNS diseases. Neurochem. Int. 2015, 89, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Golba, B.; Benetti, E.M.; De Geest, B.G. Biomaterials applications of cyclic polymers. Biomaterials 2021, 267, 120468. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Das, P.; Itzhaki, E.; Hadad, E.; Gedanken, A.; Margel, S. Microwave-synthesized polysaccharide-derived carbon dots as therapeutic cargoes and toughening agents for elastomeric gels. ACS Appl. Mater. Interfaces 2020, 46, 51940–51951. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Ganguly, S.; Margel, S.; Luong, J.H.; Gedanken, A. Applications of N-doped carbon dots as antimicrobial agents, antibiotic carriers, and selective fluorescent probes for nitro explosives. ACS Appl. Bio-Mater. 2020, 3, 8023–8031. [Google Scholar] [CrossRef]
- Yu, D.G.; Wang, M.; Ge, R. Strategies for sustained drug release from electrospun multi-layer nanostructures. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, e1772. [Google Scholar] [CrossRef]
- Guzmán-Oyarzo, D.; Plaza, T.; Recio-Sánchez, G.; Abdalla, D.S.P.; Salazar, L.A.; Hernández-Montelongo, J. Use of nPSi-βCD Composite Microparticles for the Controlled Release of Caffeic Acid and Pinocembrin, Two Main Polyphenolic Compounds Found in a Chilean Propolis. Pharmaceutics 2019, 11, 289. [Google Scholar] [CrossRef] [Green Version]
- Sinning, M.; Letelier, R.; Rosas, C.; Fuenzalida, M.; Lemus, D. Angiogenic potential of the cerebrospinal fluid (CSF) of patients with high-grade gliomas measured with the chick embryo chorioallantoic membrane assay (CAM). Biol. Res. 2012, 45, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Arnaoutova, I.; Kleinman, H.K. In vitro angiogenesis: Endothelial cell tube formation on gelled basement membrane extract. Nat. Protoc. 2010, 5, 628–635. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Mada, S.B.; Reddi, S.; Kumar, N.; Kapila, S.; Kapila, R. Protective effects of casein-derived peptide VLPVPQK against hydrogen peroxide-induced dysfunction and cellular oxidative damage in rat osteoblastic cells. Hum. Exp. Toxicol. 2017, 36, 967–980. [Google Scholar] [CrossRef]
- Bhatia, G.; Dhuna, V.; Dhuna, K.; Kaur, M.; Singh, J. Bacopa monnieri extracts prevent hydrogen peroxide-induced oxidative damage in a cellular model of neuroblastoma IMR32 cells. Chin. J. Nat. Med. 2017, 15, 834–846. [Google Scholar] [CrossRef]
- Buschmann, J.; Härter, L.; Gao, S.; Hemmi, S.; Welti, M.; Hild, N.; Schneider, O.D.; Stark, W.J.; Lindenblatt, N.; Werner, C.M.; et al. Tissue engineered bone grafts based on biomimetic nanocomposite PLGA/amorphous calcium phosphate scaffold and human adipose-derived stem cells. Injury 2012, 43, 1689–1697. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Concheiro, A.; Silva, M.; Dominguez, F.; Sheikh, F.A.; Cantu, T.; Desai, R.; Garcia, V.L.; Macossay, J. Biodegradable electrospun nanofibers coated with platelet-rich plasma for cell adhesion and proliferation. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Shafaat, S.; Mangir, N.; Regureos, S.R.; Chapple, C.R.; MacNeil, S. Demonstration of improved tissue integration and angiogenesis with an elastic, estradiol releasing polyurethane material designed for use in pelvic floor repair. Neurourol. Urodyn. 2018, 37, 716–725. [Google Scholar] [CrossRef]
- Ribatti, D.; Annese, T.; Tamma, R. The use of the chick embryo CAM assay in the study of angiogenic activiy of biomaterials. Microvasc. Res. 2020, 131, 104026. [Google Scholar] [CrossRef]
- Cacua, K.; Ordonez, F.; Zapata, C.; Herrera, B.; Pabon, E.; Buitrago-Sierra, R. Surfactant concentration and pH effects on the zeta potential values of alumina nanofluids to inspect stability. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123960. [Google Scholar] [CrossRef]
- Cutrim, C.S.; Alvim, I.D.; Cortez, M.A.S. Microencapsulation of green tea polyphenols by ionic gelation and spray chilling methods. J. Food Sci. Technol. 2019, 56, 3561–3570. [Google Scholar] [CrossRef]
- Ćorković, I.; Pichler, A.; Ivić, I.; Šimunović, J.; Kopjar, M. Microencapsulation of Chokeberry Polyphenols and Volatiles: Application of Alginate and Pectin as Wall Materials. Gels 2021, 7, 231. [Google Scholar] [CrossRef]
- Echeverria, F.; Jimenez Patino, P.A.; Castro-Sepulveda, M.; Bustamante, A.; Garcia Concha, P.A.; Poblete-Aro, C.; Valenzuela, R.; Garcia-Diaz, D.F. Microencapsulated pomegranate peel extract induces mitochondrial complex IV activity and prevents mitochondrial cristae alteration in brown adipose tissue in mice fed on a high-fat diet. Br. J. Nutr. 2021, 28, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Rahaiee, S.; Assadpour, E.; Faridi Esfanjani, A.; Silva, A.S.; Jafari, S.M. Application of nano/microencapsulated phenolic compounds against cancer. Adv. Colloid Interface Sci. 2020, 279, 102153. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Jiménez-García, M.; Capó, X.; Moranta, D.; Arnone, A.; Tenore, G.C.; Sureda, A.; Tejada, S. Microencapsulation as a tool to counteract the typical low bioavailability of polyphenols in the management of diabetes. Food Chem. Toxicol. 2020, 139, 111248. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.W.; Jiang, J.S.; Lu, W.Q. Ferulic Acid Exerts Anti-Angiogenic and Anti-Tumor Activity by Targeting Fibroblast Growth Factor Receptor 1-Mediated Angiogenesis. Int. J. Mol. Sci. 2015, 16, 24011–24031. [Google Scholar] [CrossRef]
- Ravishankar, D.; Watson, K.A.; Boateng, S.Y.; Green, R.J.; Greco, F.; Osborn, H.M. Exploring quercetin and luteolin derivatives as antiangiogenic agents. Eur. J. Med. Chem. 2015, 97, 259–274. [Google Scholar] [CrossRef]
- Cuevas, A.; Saavedra, N.; Cavalcante, M.F.; Salazar, L.A.; Abdalla, D.S. Identification of microRNAs involved in the modulation of pro-angiogenic factors in atherosclerosis by a polyphenol-rich extract from propolis. Arch. Biochem. Biophys. 2014, 557, 28–35. [Google Scholar] [CrossRef]
- Cuevas, A.; Saavedra, N.; Rudnicki, M.; Abdalla, D.S.; Salazar, L.A. ERK1/2 and HIF1α Are Involved in Antiangiogenic Effect of Polyphenols-Enriched Fraction from Chilean Propolis. Evid.-Based Complement. Altern. Med. 2015, 2015, 187575. [Google Scholar] [CrossRef] [Green Version]
- Ahn, M.R.; Kunimasa, K.; Kumazawa, S.; Nakayama, T.; Kaji, K.; Uto, Y.; Hori, H.; Nagasawa, H.; Ohta, T. Correlation between antiangiogenic activity and antioxidant activity of various components from propolis. Mol. Nutr. Food Res. 2009, 53, 643–651. [Google Scholar] [CrossRef]
- Kongpichitchoke, T.; Hsu, J.L.; Huang, T.C. Number of Hydroxyl Groups on the B-Ring of Flavonoids Affects Their Antioxidant Activity and Interaction with Phorbol Ester Binding Site of PKCδ C1B Domain: In Vitro and in Silico Studies. J. Agric. Food Chem. 2015, 63, 4580–4586. [Google Scholar] [CrossRef]
- Ushio-Fukai, M. Redox signaling in angiogenesis: Role of NADPH oxidase. Cardiovasc. Res. 2006, 71, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Sun, W.; Zhang, Z.; Zheng, Y. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid. Med. Cell Longev. 2014, 2014, 260429. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Polyphenol in Solution | Concentration (μM) | % ABTS Absorbance Inhibition | Trolox Equivalents (mg/L) |
|---|---|---|---|
| Caffeic acid | 50 | 10.7 ± 1.7 a | 1.6 ± 0.3 |
| 150 | 24.0 ± 2.7 b | 3.6 ± 0.4 | |
| 350 | 51.8 ± 5.3 c | 7.8 ± 0.8 | |
| Pinocembrin | 10 | 3.4 ± 0.6 a | 0.5 ± 0.1 |
| 20 | 3.4 ± 0.9 a | 0.5 ± 0.1 | |
| 65 | 4.8 ± 1.3 b | 0.7 ± 0.2 |
| Microencapsulated Polyphenol | % ABTS Absorbance Inhibition | Trolox Equivalents (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.5 h | 4 h | 8 h | 24 h | 0.5 h | 4 h | 8 h | 24 h | |
| CA 50 | 7.7 ± 0.6 | 6.5 ± 1.4 | 6.5 ± 0.6 | 9.0 ± 3.5 | 1.1 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.1 | 1.3 ± 0.5 |
| CA 150 | 17.8 ± 4.6 | 17.1 ± 4.6 | 24.0 ± 8.7 | 24.7 ± 8.9 | 2.6 ± 0.7 | 2.5 ± 0.7 | 3.6 ± 1.3 | 3.7 ± 1.4 |
| CA 350 | 24.3 ± 5.7 | 23.6 ± 5.7 | 48.9 ± 4.5 | 47.0 ± 4.3 | 3.6 ± 0.9 | 3.5 ± 0.9 | 7.4 ± 0.7 | 7.1 ± 0.7 |
| Pin 10 | 6.7 ± 2.0 | 5.5 ± 0.6 | 3.2 ± 0.6 | 4.7 ± 0.5 | 0.1 ± 0.3 | 0.8 ± 0.1 | 0.4 ± 0.1 | 0.7 ± 0.1 |
| Pin 20 | 6.0 ± 1.8 | 5.1 ± 0.3 | 3.9 ± 0.6 | 4.9 ± 0.3 | 0.9 ± 0.3 | 0.7 ± 0.0 | 0.5 ± 0.1 | 0.7 ± 0.1 |
| Pin 65 | 6.4 ± 1.7 | 5.8 ± 0.4 | 3.9 ± 0.4 | 6.3 ± 1.6 | 0.9 ± 0.3 | 0.8 ± 0.1 | 0.5 ± 0.1 | 0.9 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Oyarzo, D.; Hernández-Montelongo, J.; Rosas, C.; Leal, P.; Weber, H.; Alvear, M.; Salazar, L.A. Controlled Release of Caffeic Acid and Pinocembrin by Use of nPSi-βCD Composites Improves Their Antiangiogenic Activity. Pharmaceutics 2022, 14, 484. https://doi.org/10.3390/pharmaceutics14030484
Guzmán-Oyarzo D, Hernández-Montelongo J, Rosas C, Leal P, Weber H, Alvear M, Salazar LA. Controlled Release of Caffeic Acid and Pinocembrin by Use of nPSi-βCD Composites Improves Their Antiangiogenic Activity. Pharmaceutics. 2022; 14(3):484. https://doi.org/10.3390/pharmaceutics14030484
Chicago/Turabian StyleGuzmán-Oyarzo, Dina, Jacobo Hernández-Montelongo, Carlos Rosas, Pamela Leal, Helga Weber, Marysol Alvear, and Luis A. Salazar. 2022. "Controlled Release of Caffeic Acid and Pinocembrin by Use of nPSi-βCD Composites Improves Their Antiangiogenic Activity" Pharmaceutics 14, no. 3: 484. https://doi.org/10.3390/pharmaceutics14030484
APA StyleGuzmán-Oyarzo, D., Hernández-Montelongo, J., Rosas, C., Leal, P., Weber, H., Alvear, M., & Salazar, L. A. (2022). Controlled Release of Caffeic Acid and Pinocembrin by Use of nPSi-βCD Composites Improves Their Antiangiogenic Activity. Pharmaceutics, 14(3), 484. https://doi.org/10.3390/pharmaceutics14030484