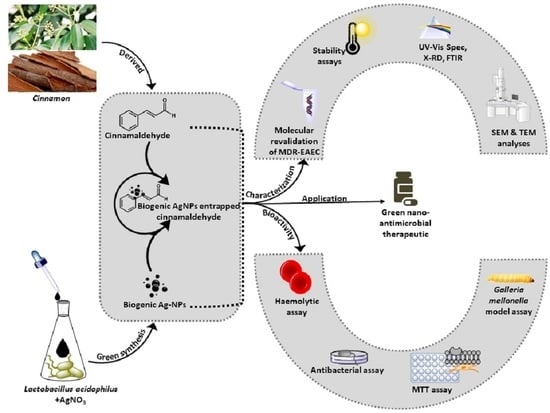
Antimicrobial Efficacy of Green Synthesized Nanosilver with Entrapped Cinnamaldehyde against Multi-Drug-Resistant Enteroaggregative Escherichia coli in Galleria mellonella
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Phytochemicals, and Nanoparticles
2.2. Entrapment of AgNPs with Cinnamaldehyde
2.3. Characterization of AgC
2.4. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.5. In Vitro Stability Assays
2.6. In Vitro Safety Assays
2.7. In Vitro Time- and Dose-Dependent Time-Kill Assay
2.8. In Vivo Antibacterial Efficacy of AgC in the G. mellonella Larval Model
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of AgC
3.2. Determination of MIC and MBC
3.3. In Vitro Stability Assays
3.4. In Vitro Safety Assays
3.5. In Vitro Time-and Dose-Dependent Time-Kill Kinetic Assay
3.6. In Vivo Antibacterial Efficacy of AgC in G. mellonella Larval Model
3.6.1. Survival Rate
3.6.2. Enumeration of MDR-EAEC Counts
3.6.3. Enumeration of Haemocytes
3.6.4. Melanization Assay
3.6.5. LDH Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joffré, E.; Iñiguez Rojas, V. Molecular epidemiology of enteroaggregative Escherichia coli (EAEC) isolates of hospitalized children from Bolivia reveal high heterogeneity and multidrug-resistance. Int. J. Mol. Sci. 2020, 21, 9543. [Google Scholar] [CrossRef]
- Lόpez-Vélez, R.; Lebens, M.; Bundy, L.; Barriga, J.; Steffen, R. Bacterial travellers’ diarrhoea: A narrative review of literature published over the past 10 year. Travel Med. Infect. Dis. 2022, 47, 102293. [Google Scholar] [CrossRef] [PubMed]
- Kantele, A.; Lääveri, T. Extended-spectrum beta-lactamase-producing strains among diarrhoeagenic Escherichia coli—Prospective traveller study with literature review. J. Travel Med. 2022, 29, taab042. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Xu, S.; Tang, Z.; Li, Z.; Zhang, L. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosaf. Health 2021, 3, 32–38. [Google Scholar] [CrossRef]
- Rezasoltani, S.; Yadegar, A.; Hatami, B.; Asadzadeh Aghdaei, H.; Zali, M.R. Antimicrobial resistance as a hidden menace lurking behind the COVID-19 outbreak: The global impacts of too much hygiene on AMR. Front. Microbiol. 2020, 11, 590683. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Haque, S.; Tripathy, S.; Patra, C. Manganese based advanced nanoparticles for biomedical applications: Future opportunity and challenges. Nanoscale 2021, 13, 16405–16426. [Google Scholar] [CrossRef]
- Zhao, Y.; Pu, M.; Zhang, J.; Wang, Y.; Yan, X.; Yu, L.; He, Z. Recent advancements of nanomaterial-based therapeutic strategies toward sepsis: Bacterial eradication, anti-inflammation, and immunomodulation. Nanoscale 2021, 13, 10726–10747. [Google Scholar] [CrossRef]
- Weldick, P.J.; Wang, A.; Halbus, A.F.; Paunov, V.N. Emerging nanotechnologies for targeting antimicrobial resistance. Nanoscale 2022, 14, 4018–4041. [Google Scholar] [CrossRef]
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M.R. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, Y.; Ran, X.; Liu, C.; Wang, Z.; Ren, J.; Qu, X. Phytochemical-encapsulated nanoplatform for “on-demand” synergistic treatment of multidrug-resistant bacteria. Nano Res. 2018, 11, 3762–3770. [Google Scholar] [CrossRef]
- Van Liefferinge, E.; Forte, C.; Degroote, J.; Ovyn, A.; Van Noten, N.; Mangelinckx, S.; Michiels, J. In vitro and in vivo antimicrobial activity of cinnamaldehyde and derivatives towards the intestinal bacteria of the weaned piglet. Ital. J. Anim. Sci. 2022, 21, 493–506. [Google Scholar] [CrossRef]
- Yu, C.; Li, Y.L.; Liang, M.; Dai, S.Y.; Ma, L.; Li, W.G.; Lai, F.; Liu, X.M. Characteristics and hazards of the cinnamaldehyde oxidation process. RSC Adv. 2020, 10, 19124–19133. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Srivastava, S.; Ghosh, S.; Khare, S.K. Phytochemical delivery through nanocarriers: A review. Colloids Surfaces B Biointerfaces 2021, 197, 111389. [Google Scholar] [CrossRef] [PubMed]
- Alkhathlan, A.H.; Al-Abdulkarim, H.A.; Khan, M.; Khan, M.; Alkholief, M.; Alshamsan, A.; Almomen, A.; Albekairi, N.; Alkhathlan, H.Z.; Siddiqui, M.R.H. Evaluation of the anticancer activity of phytomolecules conjugated gold nanoparticles synthesized by squeous extracts of Zingiber officinale (ginger) and Nigella sativa L. seeds (black cumin). Materials 2021, 14, 3368. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Hakeem, K.R.; Rehman, R.U. Synergistic effect of plant extract coupled silver nanoparticles in various therapeutic applications- present insights and bottlenecks. Chemosphere 2022, 288, 132527. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, J.; Wang, Y.; Chen, C.; Gu, H.; Chai, Y.; Wang, Y. Silver nanoparticles-decorated and mesoporous silica coated single-walled carbon nanotubes with an enhanced antibacterial activity for killing drug-resistant bacteria. Nano Res. 2020, 13, 389–400. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Strong antimicrobial activity of silver nanoparticles obtained by the green synthesis in Viridibacillus sp. extracts. Front. Microbiol. 2022, 13, 820048. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Rajan, R.; Huo, P.; Chandran, K.; Dakshinamoorthi, B.M.; Yun, S.-I.; Liu, B. A review on the toxicity of silver nanoparticles against different biosystems. Chemosphere 2022, 292, 133397. [Google Scholar] [CrossRef]
- Fatima, F.; Aldawsari, M.F.; Ahmed, M.M.; Anwer, M.K.; Naz, M.; Ansari, M.J.; Hamad, A.M.; Zafar, A.; Jafar, M. Green synthesized silver nanoparticles using Tridax procumbens for topical application: Excision wound model and histopathological studies. Pharmaceutics 2021, 13, 1754. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.; Sibuyi, N.R.S.; Fadaka, A.O.; Madiehe, M.A.; Ernest Maboza, M.M.; Geerts, G. Plant extract-synthesized silver nanoparticles for application in dental therapy. Pharmaceutics 2022, 14, 380. [Google Scholar] [CrossRef] [PubMed]
- Alahmad, A.; Al-Zereini, W.A.; Hijazin, T.J.; Al-Madanat, O.Y.; Alghoraibi, I.; Al-Qaralleh, O.; Al-Qaraleh, S.; Feldhoff, A.; Walter, J.-G.; Scheper, T. Green synthesis of silver nanoparticles using Hypericum perforatum L. aqueous extract with the evaluation of its antibacterial activity against clinical and food pathogens. Pharmaceutics 2022, 14, 1104. [Google Scholar] [CrossRef] [PubMed]
- Abishad, P.; Vergis, J.; Unni, V.; Ram, V.P.; Niveditha, P.; Yasur, J.; Juliet, S.; John, L.; Byrappa, K.; Nambiar, P.; et al. Green synthesized silver nanoparticles using Lactobacillus acidophilus as an antioxidant, antimicrobial, and antibiofilm agent against multi-drug resistant Enteroaggregative Escherichia coli. Probiotics Antimicrob. Proteins 2022, 1–11. [Google Scholar] [CrossRef]
- Vergis, J.; Malik, S.S.; Pathak, R.; Kumar, M.; Ramanjaneya, S.; Kurkure, N.V.; Barbuddhe, S.B.; Rawool, D.B. Exploiting lactoferricin (17-30) as a potential antimicrobial and antibiofilm candidate against multi-drug-resistant enteroaggregative Escherichia coli. Front. Microbiol. 2020, 11, 575917. [Google Scholar] [CrossRef]
- Vergis, J.; Malik, S.S.; Pathak, R.; Kumar, M.; Ramanjaneya, S.; Kurkure, N.V.; Barbuddhe, S.B.; Rawool, D.B. Antimicrobial efficacy of indolicidin against multi-drug resistant enteroaggregative Escherichia coli in a Galleria mellonella model. Front. Microbiol. 2019, 10, 2723. [Google Scholar] [CrossRef]
- Vergis, J.; Malik, S.V.S.; Pathak, R.; Kumar, M.; Kurkure, N.V.; Barbuddhe, S.B.; Rawool, D.B. Exploring Galleria mellonella larval model to evaluate antibacterial efficacy of Cecropin A (1-7)-melittin against multi-drug resistant enteroaggregative Escherichia coli. Pathog. Dis. 2021, 79, ftab010. [Google Scholar] [CrossRef]
- Blasco, L.; Ambroa, A.; Trastoy, R.; Bleriot, I.; Moscoso, M.; Fernández-Garcia, L.; Perez-Nadales, E.; Fernández-Cuenca, F.; Torre-Cisneros, J.; Oteo-Iglesias, J.; et al. In vitro and in vivo efficacy of combinations of colistin and different endolysins against clinical strains of multi-drug resistant pathogens. Sci. Rep. 2020, 10, 7163. [Google Scholar] [CrossRef]
- Junqueira, J.C.; Mylonakis, E.; Borghi, E. Galleria mellonella experimental model: Advances and future directions. Pathog. Dis. 2021, 79, ftab021. [Google Scholar] [CrossRef]
- Curtis, A.; Binder, U.; Kavanagh, K. Galleria mellonella larvae as a model for investigating host-fungal interactions. Front. Fungal Biol. 2022, 3, 893494. [Google Scholar] [CrossRef]
- Abishad, P.; Niveditha, P.; Unni, V.; Vergis, J.; Kurkure, N.V.; Chaudhari, S.; Rawool, D.B.; Barbuddhe, S.B. In silico molecular docking and in vitro antimicrobial efficacy of phytochemicals against multi-drug-resistant enteroaggregative Escherichia coli and non-typhoidal Salmonella spp. Gut Pathog. 2021, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Aljaafari, M.N.; Alkhoori, M.A.; Hag-Ali, M.; Cheng, W.H.; Lim, S.H.E.; Loh, J.Y.; Lai, K.S. Contribution of aldehydes and their derivatives to antimicrobial and immunomodulatory activities. Molecules 2022, 27, 3589. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, I.F.; Araújo, L.G.; Assunção, R.G.; Dutra, I.L.; Nascimento, J.R.; Rego, F.S.; Rolim, C.S.; Alves, L.S.; Frazão, M.A.; Cadete, S.F.; et al. Cinnamaldehyde increases the survival of mice submitted to sepsis induced by extraintestinal pathogenic Escherichia coli. Antibiotics 2022, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Vijay, D.; Dhaka, P.; Vergis, J.; Negi, M.; Mohan, V.; Kumar, M.; Doijad, S.; Poharkar, K.; Kumar, A.; Malik, S.S.; et al. Characterization and biofilm forming ability of diarrhoeagenic enteroaggregative Escherichia coli isolates recovered from human infants and young animals. Comp. Immunol. Microbiol. Infect. Dis. 2015, 38, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Mioc, M.; Pavel, I.Z.; Ghiulai, R.; Coricovac, D.E.; Farcaş, C.; Mihali, C.-V.; Oprean, C.; Serafim, V.; Popovici, R.A.; Dehelean, C.A.; et al. The cytotoxic effects of betulin conjugated gold nanoparticles as stable formulations in normal and melanoma cells. Front. Pharmacol. 2018, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; DeTrizio, A.; Spalla, M.; Migliavacca, R.; Pagani, L.; Pisani, S.; Chiesa, E.; Conti, B.; Modena, T.; Genta, I. Gentamicin sulfate PEG-PLGA/PLGA-H nanoparticles: Screening design and antimicrobial effect evaluation toward clinic bacterial isolates. Nanomaterials 2018, 8, 37. [Google Scholar] [CrossRef]
- CLSI Performance Standards for Antimicrobial Susceptibility Testing, 32th Edition. Available online: https://clsi.org/standards/products/microbiology/d (accessed on 20 April 2022).
- Gourkhede, D.P.; Bhoomika, S.; Pathak, R.; Yadav, J.P.; Nishanth, D.; Vergis, J.; Malik, S.V.S.; Barbuddhe, S.B.; Rawool, D.B. Antimicrobial efficacy of Cecropin A (1–7)- Melittin and Lactoferricin (17–30) against multi-drug resistant Salmonella Enteritidis. Microb. Pathog. 2020, 147, 104405. [Google Scholar] [CrossRef]
- Nishanth, M.A.D.; Bhoomika, S.; Gourkhede, D.; Dadimi, B.; Vergis, J.; Malik, S.V.S.; Barbuddhe, S.B.; Rawool, D.B. Antibacterial efficacy of in-house designed cell-penetrating peptide against multi-drug resistant strains of Salmonella Enteritidis and Salmonella Typhimurium. Environ. Microbiol. 2022, 24, 2747–2758. [Google Scholar] [CrossRef]
- Cabrera-Sosa, L.; Ochoa, T.J. Escherichia coli diarrhea. In Hunter’s Tropical Medicine and Emerging Infectious Diseases; Ryan, E.T., Hill, D.R., Solmon, T., Aronson, N.E., Endy, T.P., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 481–485. [Google Scholar]
- Abdelwahab, R.; Yasir, M.; Godfrey, R.E.; Christie, G.S.; Element, S.J.; Saville, F.; Hassan, E.A.; Ahmed, E.H.; Abu-Faddan, N.H.; Daef, E.A.; et al. Antimicrobial resistance and gene regulation in Enteroaggregative Escherichia coli from Egyptian children with diarrhoea: Similarities and differences. Virulence 2021, 12, 57–74. [Google Scholar] [CrossRef]
- Ellis, S.J.; Crossman, L.C.; McGrath, C.J.; Chattaway, M.A.; Hölken, J.M.; Brett, B.; Bundy, L.; Kay, G.L.; Wain, J.; Schüller, S. Identification and characterisation of enteroaggregative Escherichia coli subtypes associated with human disease. Sci. Rep. 2020, 10, 7475. [Google Scholar] [CrossRef]
- Kashima, K.; Sato, M.; Osaka, Y.; Sakakida, N.; Kando, S.; Ohtsuka, K.; Doi, R.; Chiba, Y.; Takase, S.; Fujiwara, A.; et al. An outbreak of food poisoning due to Escherichia coli serotype O7:H4 carrying astA for enteroaggregative E. coli heat-stable enterotoxin1 (EAST1). Epidemiol. Infect. 2021, 149, e244. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Santos, V.I.; Ruíz-Rosas, M.; Ramirez-Peralta, A.; García, O.Z.; Resendiz-Reyes, L.A.; Romero-Pineda, O.J.; Castro-Alarcón, N. Enteroaggregative Escherichia coli is associated with antibiotic resistance and urinary tract infection symptomatology. PeerJ 2021, 9, e11726. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Kumar, S.H.; Girisha, S.K.; Shetty, A.V. Characterization of virulence gene distribution and antibiotic susceptibility profiles of diarrheagenic Escherichia coli from chicken faeces. J. Adv. Biotechnol. Exp. Ther. 2022, 5, 148–162. [Google Scholar] [CrossRef]
- Lawal, O.U.; Parreira, V.R.; Goodridge, L. The biology and the evolutionary dynamics of diarrheagenic Escherichia coli pathotypes. In Escherichia coli; Erjavec, M.S., Ed.; IntechOpen: London, UK, 2022; pp. 1–37. [Google Scholar]
- Elshaghabee, F.M.; Rokana, N. Dietary management by probiotics, prebiotics and synbiotics for the prevention of antimicrobial resistance. In Sustainable Agriculture Reviews 49; Panwar, H., Sharma, C., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2021; pp. 33–56. [Google Scholar]
- Streicher, L.M. Exploring the future of infectious disease treatment in a post-antibiotic era: A comparative review of alternative therapeutics. J. Glob. Antimicrob. Resist. 2021, 24, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, L.; Wang, Y.; Song, X.; Li, K.; Yan, X.; Yu, L.; He, Z. Nanomaterial-based strategies in antimicrobial applications: Progress and perspective. Nano Res. 2021, 14, 4417–4441. [Google Scholar] [CrossRef]
- Cox, H.J.; Li, J.; Saini, P.; Paterson, J.R.; Sharples, G.J.; Badyal, J.P.S. Bioinspired and eco-friendly high efficacy cinnamaldehyde antibacterial surfaces. J. Mater. Chem. B. 2021, 9, 2918–2930. [Google Scholar] [CrossRef]
- Jemal, K.; Sandeep, B.V.; Pola, S. Synthesis, characterization, and evaluation of the antibacterial activity of Allophylus serratus leaf and leaf derived callus extracts mediated silver nanoparticles. J. Nanomater. 2017, 2017, 4213275. [Google Scholar] [CrossRef]
- Chandran, S.; Ravichandran, V.; Chandran, S.; Chemmanda, J.; Chandarshekar, B. Biosynthesis of PVA encapsulated silver nanoparticles. J. Appl. Res. Technol. 2016, 14, 319–324. [Google Scholar] [CrossRef]
- El-Moslamy, S.H.; Elkady, M.F.; Rezk, A.H.; Abdel-Fattah, Y.R. Applying Taguchi design and large-scale strategy for mycosynthesis of nano-silver from endophytic Trichoderma harzianum SYA. F4 and its application against phytopathogen. Sci. Rep. 2017, 7, 45297. [Google Scholar] [CrossRef]
- Yang, Z.; He, S.; Wu, H.; Yin, T.; Wang, L.; Shan, A. Nanostructured antimicrobial peptides: Crucial steps of overcoming the bottleneck for clinics. Front. Microbiol. 2021, 12, 710199. [Google Scholar] [CrossRef]
- Pereira, W.A.; Pereira, C.D.S.; Assunção, R.G.; da Silva, I.S.C.; Rego, F.S.; Alves, L.S.; Santos, J.S.; Nogueira, F.J.R.; Zagmignan, A.; Thomsen, T.T.; et al. New insights into the antimicrobial action of Cinnamaldehyde towards Escherichia coli and its effects on intestinal colonization of mice. Biomolecules 2021, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Urnukhsaikhan, E.; Bold, B.E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef] [PubMed]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green synthesized silver nanoparticles: Antibacterial and anticancer activities, biocompatibility, and analyses of surface-attached proteins. Front. Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef] [PubMed]
- Lowry, C.A. Characterization of a Putative Molecular Activator of Spreading Depolarization Generated by the Ischemic Brain. Ph.D. Thesis, Queen’s University, Kingston, ON, Canada, 2022. [Google Scholar]
- Chen, L.Q.; Fang, L.; Ling, J.; Ding, C.Z.; Kang, B.; Huang, C.Z. Nanotoxicity of silver nanoparticles to red blood cells: Size dependent adsorption, uptake, and hemolytic activity. Chem. Res. Toxicol. 2015, 28, 501–509. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garnæs, J.; Mijakovic, I. A sustainable approach for the green synthesis of silver nanoparticles from Solibacillus isronensis sp. and their application in biofilm inhibition. Molecules 2020, 25, 2783. [Google Scholar] [CrossRef]
- Jonsson, R.; Struve, C.; Jenssen, H.; Krogfelt, K.A. The wax moth Galleria mellonella as a novel model system to study Enteroaggregative Escherichia coli pathogenesis. Virulence 2017, 8, 1894–1899. [Google Scholar] [CrossRef]
- Piatek, M.; Sheehan, G.; Kavanagh, K. Galleria mellonella: The versatile Host for drug discovery, in vivo toxicity testing and characterising host-pathogen interactions. Antibiotics 2021, 10, 1545. [Google Scholar] [CrossRef]
- Dijokaite, A.; Humbert, M.V.; Borkowski, E.; La Ragione, R.M.; Christodoulides, M. Establishing an invertebrate Galleria mellonella greater wax moth larval model of Neisseria gonorrhoeae infection. Virulence 2021, 12, 1900–1920. [Google Scholar] [CrossRef]
- Thomaz, L.; Gustavo de Almeida, L.; Silva, F.R.; Cortez, M.; Taborda, C.P.; Spira, B. In vivo activity of silver nanoparticles against Pseudomonas aeruginosa infection in Galleria mellonella. Front. Microbiol. 2020, 11, 582107. [Google Scholar] [CrossRef]
- Campo-Beleño, C.; Villamizar-Gallardo, R.A.; López-Jácome, L.E.; González, E.E.; Muñoz-Carranza, S.; Franco, B.; Morales-Espinosa, R.; Coria-Jimenez, R.; Franco-Cendejas, R.; Hernández-Durán, M.; et al. Biologically synthesized silver nanoparticles as potent antibacterial effective against multi drug-resistant Pseudomonas aeruginosa. Lett. Appl. Microbiol. 2022. [Google Scholar] [CrossRef]
- Ferro, T.A.F.; Araújo, J.M.M.; Pinto, B.L.D.S.; Dos Santos, J.S.; Souza, E.B.; da Silva, B.L.R.; Colares, V.L.P.; Novais, T.M.G.; Filho, C.M.B.; Struve, C.; et al. Cinnamaldehyde inhibits Staphylococcus aureus virulence factors and protects against infection in a Galleria mellonella model. Front. Microbiol. 2016, 7, 2052. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Venkitanarayanan, K. In vivo efficacy of trans-cinnamaldehyde, carvacrol, and thymol in attenuating Listeria monocytogenes infection in a Galleria mellonella model. J. Nat. Med. 2016, 70, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.J.; Sousa, F.I.; Pereira, D.M.; Ferro, T.A.; Pereira, I.C.; Silva, B.L.; Pinheiro, A.J.; Mouchrek, A.Q.; Monteiro-Neto, V.; Costa, S.K.; et al. Cinnamaldehyde modulates LPS-induced systemic inflammatory response syndrome through TRPA1-dependent and independent mechanisms. Int. Immunopharmacol. 2016, 34, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Moya-Andérico, L.; Vukomanovic, M.; del Mar Cendra, M.; Segura-Feliu, M.; Gil, V.; José, A.; Torrents, E. Utility of Galleria mellonella larvae for evaluating nanoparticle toxicology. Chemosphere 2021, 266, 129235. [Google Scholar] [CrossRef]
- Coates, C.J.; Lim, J.; Harman, K.; Rowley, A.F.; Griffiths, D.J.; Emery, H.; Layton, W. The insect, Galleria mellonella, is a compatible model for evaluating the toxicology of okadaic acid. Cell Biol. Toxicol. 2019, 35, 219–232. [Google Scholar] [CrossRef] [Green Version]





| Isolate | Cinnamaldehyde | AgNPs | AgC | |||
|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| MDR-1 | 0.256 | 0.512 | 0.008 | 0.016 | 0.008 | 0.008 |
| MDR-2 | 0.256 | 0.256 | 0.016 | 0.032 | 0.008 | 0.016 |
| MDR-3 | 0.512 | 0.512 | 0.016 | 0.032 | 0.016 | 0.032 |
| Concentration | Haemolysis (%) | ||
|---|---|---|---|
| Cinnamaldehyde | AgNPs | AgC | |
| MIC (1X) | 3.61 | 0.348 | 0.208 |
| MIC (2X) | 4.60 | 0.856 | 0.682 |
| MIC (5X) | 9.80 | 1.53 | 0.905 |
| MIC (10X) | 12.62 | 2.57 | 3.064 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasastha Ram, V.; Yasur, J.; Abishad, P.; Unni, V.; Purushottam Gourkhede, D.; Nishanth, M.A.D.; Niveditha, P.; Vergis, J.; Singh Malik, S.V.; Kullaiah, B.; et al. Antimicrobial Efficacy of Green Synthesized Nanosilver with Entrapped Cinnamaldehyde against Multi-Drug-Resistant Enteroaggregative Escherichia coli in Galleria mellonella. Pharmaceutics 2022, 14, 1924. https://doi.org/10.3390/pharmaceutics14091924
Prasastha Ram V, Yasur J, Abishad P, Unni V, Purushottam Gourkhede D, Nishanth MAD, Niveditha P, Vergis J, Singh Malik SV, Kullaiah B, et al. Antimicrobial Efficacy of Green Synthesized Nanosilver with Entrapped Cinnamaldehyde against Multi-Drug-Resistant Enteroaggregative Escherichia coli in Galleria mellonella. Pharmaceutics. 2022; 14(9):1924. https://doi.org/10.3390/pharmaceutics14091924
Chicago/Turabian StylePrasastha Ram, Vemula, Jyothsna Yasur, Padikkamannil Abishad, Varsha Unni, Diksha Purushottam Gourkhede, Maria Anto Dani Nishanth, Pollumahanti Niveditha, Jess Vergis, Satya Veer Singh Malik, Byrappa Kullaiah, and et al. 2022. "Antimicrobial Efficacy of Green Synthesized Nanosilver with Entrapped Cinnamaldehyde against Multi-Drug-Resistant Enteroaggregative Escherichia coli in Galleria mellonella" Pharmaceutics 14, no. 9: 1924. https://doi.org/10.3390/pharmaceutics14091924
APA StylePrasastha Ram, V., Yasur, J., Abishad, P., Unni, V., Purushottam Gourkhede, D., Nishanth, M. A. D., Niveditha, P., Vergis, J., Singh Malik, S. V., Kullaiah, B., Kurkure, N. V., Ramesh, C., Dufossé, L., Rawool, D. B., & Barbuddhe, S. B. (2022). Antimicrobial Efficacy of Green Synthesized Nanosilver with Entrapped Cinnamaldehyde against Multi-Drug-Resistant Enteroaggregative Escherichia coli in Galleria mellonella. Pharmaceutics, 14(9), 1924. https://doi.org/10.3390/pharmaceutics14091924