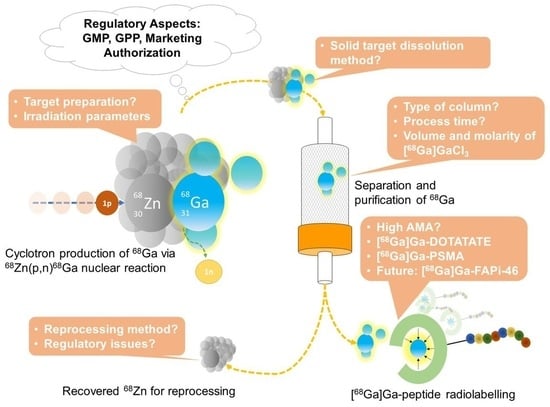
Cyclotron Production of Gallium-68 Radiopharmaceuticals Using the 68Zn(p,n)68Ga Reaction and Their Regulatory Aspects
Abstract
:1. Introduction
2. Cyclotron-Produced 68Ga Using a Solid Target
2.1. 68Ga Production Using a Solid Target in a Medical Scale PETtrace Cyclotron (GE Healthcare, Milwaukee, WI, USA)
2.2. 68Ga Production Using a Solid Target with ACSI Cyclotron
2.3. Solid Target Dissolution, Target Material (68Zn) Separation and Purification, and 68GaCl3 Formulation
3. Cyclotron-Produced 68Ga Using a Liquid Target
4. Matters in 68Ga Cyclotron Production
4.1. Expansion of Solid Target 68Ga Preparations
4.2. Sustainable Practice in Cyclotron-Produced 68Ga: [68Zn]ZnO Target Reprocessing
4.3. Optimization of 68Ga Radiopharmaceutical Production via a Liquid Target
5. Regulatory Aspects of Cyclotron-Produced 68Ga Radiopharmaceuticals
5.1. Target Transfer System and Processing
5.2. Chemical Preparations
5.3. Synthesis Module Supervision Software
5.4. Quality Control of 68Ga Radiopharmaceuticals
5.5. Metal Testing for [68Ga]GaCl3
5.6. Process Validation
5.7. Other Consideration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, S.R.; Pomper, M. Clinical applications of Gallium-68. Appl. Radiat. Isot. 2013, 76, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breeman, W.A.; de Blois, E.; Sze Chan, H.; Konijnenberg, M.; Kwekkeboom, D.J.; Krenning, E.P. 68Ga-labeled DOTA-peptides and 68Ga-labeled radiopharmaceuticals for positron emission tomography: Current status of research, clinical applications, and future perspectives. Semin. Nucl. Med. 2011, 41, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Maecke, H.; Börner, R.; Weckesser, E.; Schöffski, P.; Oei, L.; Schumacher, J.; Henze, M.; Heppeler, A.; Meyer, J. Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: Preliminary data. Eur. J. Nucl. Med. 2001, 28, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Raj, N.; Reidy-Lagunes, D. The Role of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography in Well-Differentiated Neuroendocrine Tumors: A Case-Based Approach Illustrates Potential Benefits and Challenges. Pancreas 2018, 47, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ashhar, Z.; Yusof, N.A.; Ahmad Saad, F.F.; Mohd Nor, S.M.; Mohammad, F.; Bahrin Wan Kamal, W.H.; Hassan, M.H.; Ahmad Hassali, H.; Al-Lohedan, H.A. Preparation, Characterization, and Radiolabeling of [68Ga]Ga-NODAGA-Pamidronic Acid: A Potential PET Bone Imaging Agent. Molecules 2020, 25, 2668. [Google Scholar] [CrossRef]
- FDA Approves Netspot to Detect Rare Neuroendocrine Tumors. Oncol. Times 2016, 38, 7. [CrossRef]
- FDA. FDA Letter of Approval for [68Ga]Ga-DOTA-TOC. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2019/210828Orig1s000ltr.pdf (accessed on 28 November 2022).
- Gasch, C.; Düwel, C.; Kopka, K.; Kratochwil, C.; Vinsensia, M.; Eiber, M.; Maurer, T.; Haberkorn, U.; Hadaschik, B.; Giesel, F.L. Significance of PSMA imaging in prostate cancer. Der Urol. 2017, 56, 3–12. [Google Scholar] [CrossRef]
- Ceci, F.; Fanti, S. PSMA-PET/CT imaging in prostate cancer: Why and when. Clin. Transl. Imaging 2019, 7, 377–379. [Google Scholar] [CrossRef] [Green Version]
- von Eyben, F.E.; Baumann, G.; Baum, R. PSMA diagnostics and treatments of prostate cancer become mature. Clin. Transl. Imaging 2018, 6, 145–148. [Google Scholar] [CrossRef] [Green Version]
- FDA. FDA Letter of Approval for [68Ga]Ga-PSMA-11; FDA: Silver Spring, MD, USA, 2020.
- Pichler, B.J.; Wehrl, H.; Judenhofer, M. Latest Advances in Molecular Imaging Instrumentation. J. Nucl. Med. 2008, 49 (Suppl. 2), 5S–23S. [Google Scholar] [CrossRef]
- Jakoby, B.W.; Bercier, Y.; Conti, M.; Casey, M.E.; Bendriem, B.; Townsend, D.W. Physical and clinical performance of the mCT time-of-flight PET/CT scanner. Phys. Med. Biol. 2011, 56, 2375–2389. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.H.; Din, A.T.U.; Khan, A. Neuroendocrine Tumor Therapy with Lutetium-177: A Literature Review. Cureus 2019, 11, e3986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmett, L.; Willowson, K.; Violet, J.; Shin, J.; Blanksby, A.; Lee, J. Lutetium 177 PSMA radionuclide therapy for men with prostate cancer: A review of the current literature and discussion of practical aspects of therapy. J. Med. Radiat. Sci. 2017, 64, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Iravani, A.; Violet, J.; Azad, A.; Hofman, M.S. Lutetium-177 prostate-specific membrane antigen (PSMA) theranostics: Practical nuances and intricacies. Prostate Cancer Prostatic Dis. 2020, 23, 38–52. [Google Scholar] [CrossRef]
- Kumar, K. The Current Status of the Production and Supply of Gallium-68. Cancer Biother. Radiopharm. 2020, 35, 163–166. [Google Scholar] [CrossRef]
- Synowiecki, M.A.; Perk, L.; Nijsen, J. Production of novel diagnostic radionuclides in small medical cyclotrons. EJNMMI Radiopharm. Chem. 2018, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Qaim, S.M. Theranostic radionuclides: Recent advances in production methodologies. J. Radioanal. Nucl. Chem. 2019, 322, 1257–1266. [Google Scholar] [CrossRef]
- Bandoli, G.; Dolmella, A.; Tisato, F.; Porchia, M.; Refosco, F. Mononuclear six-coordinated Ga(III) complexes: A comprehensive survey. Coord. Chem. Rev. 2009, 253, 56–77. [Google Scholar] [CrossRef]
- Szelecsényi, F.; Kovács, Z.; Nagatsu, K.; Fukumura, K.; Suzuki, K.; Mukai, K. Investigation of direct production of 68Ga with low energy multiparticle accelerator. Radiochim. Acta 2012, 100, 5–11. [Google Scholar] [CrossRef]
- Lin, M.; Waligorski, G.; Lepera, C. Production of curie quantities of 68Ga with a medical cyclotron via the 68Zn(p,n)68Ga reaction. Appl. Radiat. Isot. 2018, 133, 1–3. [Google Scholar] [CrossRef]
- Aslam, M.T.; Ali, W.; Hussain, M. Nuclear model analysis of the 65Cu(α, n)68Ga reaction for the production of 68Ga up to 40 MeV. Appl. Radiat. Isot. 2021, 170, 109590. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.N.; Amjed, N.; Qaim, S. Evaluation of excitation functions of the 68,67,66Zn(p,xn)68,67,66Ga and 67Zn(p,α)64Cu reactions: Validation of evaluated data through comparison with experimental excitation functions of the natZn(p,x)66,67Ga and natZn(p,x)64Cu processes. Appl. Radiat. Isot. 2015, 96, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Kakavand, T.; Rajabifar, S.; Mokhtari, L.; Rahimi-Nezhad, A. Cyclotron production of 68Ga via proton-induced reaction on 68Zn target. Nukleonika 2009, 54, 25–28. [Google Scholar]
- Engle, J.W.; Lopez-Rodriguez, V.; Gaspar-Carcamo, R.E.; Valdovinos, H.F.; Valle-Gonzalez, M.; Trejo-Ballado, F.; Severin, G.W.; Barnhart, T.E.; Nickles, R.J.; Avila-Rodriguez, M.A. Very high specific activity 66/68Ga from zinc targets for PET. Appl. Radiat. Isot. 2012, 70, 1792–1796. [Google Scholar] [CrossRef] [Green Version]
- Nelson, B.J.B.; Nelson, B.J.B.; Wilson, J.; Richter, S.; Duke, M.J.M.; Wuest, M.; Wuest, F. Taking cyclotron 68Ga production to the next level: Expeditious solid target production of 68Ga for preparation of radiotracers. Nucl. Med. Biol. 2020, 80, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Hesselmann, R.; Oezdemir, U.; Bertschi, L.; Blanc, A.; Dragic, M.; Löffler, D.; Smuda, C.; Johayem, A.; Schibli, R. Identification, characterization and suppression of side-products formed during the synthesis of high dose 68Ga-DOTA-TATE. Appl. Radiat. Isot. 2013, 76, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Breeman, W.A.; Klette, I.; Gottschaldt, M.; Odparlik, A.; Baehre, M.; Tworowska, I.; Schultz, M.K. Radiolabeling of DOTA-like conjugated peptides with generator-produced 68Ga and using NaCl-based cationic elution method. Nat. Protoc. 2016, 11, 1057–1066. [Google Scholar] [CrossRef]
- Luurtsema, G.; Pichler, V.; Bongarzone, S.; Seimbille, Y.; Elsinga, P.; Gee, A.; Vercouillie, J. EANM guideline for harmonisation on molar activity or specific activity of radiopharmaceuticals: Impact on safety and imaging quality. EJNMMI Radiopharm. Chem. 2021, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Coenen, H.H.; Gee, A.D.; Adam, M.; Antoni, G.; Cutler, C.S.; Fujibayashi, Y.; Jeong, J.M.; Mach, R.H.; Mindt, T.L.; Pike, V.W.; et al. Consensus nomenclature rules for radiopharmaceutical chemistry—Setting the record straight. Nucl. Med. Biol. 2017, 55, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Thisgaard, H.; Kumlin, J.; Langkjær, N.; Chua, J.; Hook, B.; Jensen, M.; Kassaian, A.; Zeisler, S.; Borjian, S.; Cross, M.; et al. Multi-curie production of gallium-68 on a biomedical cyclotron and automated radiolabelling of PSMA-11 and DOTATATE. EJNMMI Radiopharm. Chem. 2021, 6, 1. [Google Scholar] [CrossRef]
- Alnahwi, A.H.; Tremblay, S.; Ait-Mohand, S.; Beaudoin, J.F.; Guérin, B. Automated radiosynthesis of 68Ga for large-scale routine production using 68Zn pressed target. Appl. Radiat. Isot. 2020, 156, 109014. [Google Scholar] [CrossRef] [PubMed]
- Siikanen, J.; Jussing, E.; Milton, S.; Steiger, C.; Ulin, J.; Jonsson, C.; Samén, E.; Tran, T.A. Cyclotron-produced 68Ga from enriched 68Zn foils. Appl. Radiat. Isot. 2021, 176, 109825. [Google Scholar] [CrossRef] [PubMed]
- Riga, S.; Cicoria, G.; Pancaldi, D.; Zagni, F.; Vichi, S.; Dassenno, M.; Mora, L.; Lodi, F.; Morigi, M.P.; Marengo, M. Production of Ga-68 with a General Electric PETtrace cyclotron by liquid target. Phys. Med. Eur. J. Med. Phys. 2018, 55, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Byrne, J.F.; Schlasner, K.N.; Schmit, N.R.; DeGrado, T.R. Cyclotron production of 68Ga in a liquid target: Effects of solution composition and irradiation parameters. Nucl. Med. Biol. 2019, 74, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Rodnick, M.E.; Sollert, C.; Stark, D.; Clark, M.; Katsifis, A.; Hockley, B.G.; Parr, D.C.; Frigell, J.; Henderson, B.D.; Abghari-Gerst, M.; et al. Cyclotron-based production of 68Ga, [68Ga]GaCl3, and [68Ga]Ga-PSMA-11 from a liquid target. EJNMMI Radiopharm. Chem. 2020, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Tieu, W.; Hollis, C.A.; Kuan, K.K.W.; Takhar, P.; Stuckings, M.; Spooner, N.; Malinconico, M. Rapid and automated production of [68Ga]gallium chloride and [68Ga]Ga-DOTA-TATE on a medical cyclotron. Nucl. Med. Biol. 2019, 74, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Svedjehed, J.; Pärnaste, M.; Gagnon, K. Demystifying solid targets: Simple and rapid distribution-scale production of [68Ga]GaCl3 and [68Ga]Ga-PSMA-11. Nucl. Med. Biol. 2022, 104, 1–10. [Google Scholar] [CrossRef]
- Seemann, J.; Eppard, E.; Waldron, B.P.; Ross, T.L.; Roesch, F. Cation exchange-based post-processing of 68Ga-eluate: A comparison of three solvent systems for labelling of DOTATOC, NO2APBP and DATAm. Appl. Radiat. Isot. 2015, 98, 54–59. [Google Scholar] [CrossRef]
- Larenkov, A.A.; Bruskin, A.; Kodina, G. Preparation of highly purified 68Ga solutions via ion exchange in hydrochloric acid–ethanol mixtures. J. Radioanal. Nucl. Chem. 2015, 305, 147–160. [Google Scholar] [CrossRef]
- Eppard, E.; Wuttke, M.; Nicodemus, P.L.; Rösch, F. Ethanol-based post-processing of generator-derived 68Ga Toward kit-type preparation of 68Ga-radiopharmaceuticals. J. Nucl. Med. 2014, 55, 1023–1028. [Google Scholar] [CrossRef] [Green Version]
- Rösch, F. Post-processing via cation exchange cartridges: Versatile options. In Theranostics, Gallium-68, and Other Radionuclides. Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 2013; pp. 33–42. [Google Scholar]
- Zoller, F.; Riss, P.J.; Montforts, F.-P.; Rösch, F. Efficient post-processing of aqueous generator eluates facilitates 68Ga-labelling under anhydrous conditions. Radiochim. Acta 2010, 98, 157–160. [Google Scholar] [CrossRef]
- Antuganov, D.O.; Ryzhkova, D.V.; Timofeev, V.V.; Zykova, T.A.; Antuganova, Y.O.; Timofeeva, K.Y.; Samburov, O.P.; Zykov, M.P. Modification of an Anion-Exchange Procedure for 68Ga Preconcentration and Automated Synthesis of [68Ga]Ga-PSMA-11. Radiochemistry 2019, 61, 748–753. [Google Scholar] [CrossRef]
- Lin, M.; Paolillo, V.; Ta, R.T.; Damasco, J.; Rojo, R.D.; Carl, J.C.; Melancon, M.P.; Ravizzini, G.C.; Le, D.B.; Santos, E.B. Fully automated preparation of 68Ga-PSMA-11 at curie level quantity using cyclotron-produced 68Ga for clinical applications. Appl. Radiat. Isot. 2020, 155, 108936. [Google Scholar] [CrossRef] [PubMed]
- Meisenheimer, M.; Kürpig, S.; Essler, M.; Eppard, E. Ethanol effects on 68Ga-radiolabelling efficacy and radiolysis in automated synthesis utilizing NaCl post-processing. EJNMMI Radiopharm. Chem. 2019, 4, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velikyan, I. 68Ga-Based radiopharmaceuticals: Production and application relationship. Molecules 2015, 20, 12913–12943. [Google Scholar] [CrossRef] [Green Version]
- Eppard, E.; Pèrez-Malo, M.; Rösch, F. Improved radiolabeling of DOTATOC with trivalent radiometals for clinical application by addition of ethanol. EJNMMI Radiopharm. Chem. 2017, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Alves, F.; Alves, V.H.P.; Do Carmo, S.J.C.; Neves, A.C.B.; Silva, M.; Abrunhosa, A.J. Production of copper-64 and gallium-68 with a medical cyclotron using liquid targets. Mod. Phys. Lett. A 2017, 32, 1740013. [Google Scholar] [CrossRef]
- Pandey, M.K.; Byrne, J.F.; Jiang, H.; Packard, A.B.; DeGrado, T.R. Cyclotron production of 68Ga via the 68Zn(P N)68Ga Reaction in Aqueous Solution. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 303–310. [Google Scholar]
- Jensen, M.; Clark, J.C. Direct production of Ga-68 from proton bombardment of concentrated aqueous solutions of [Zn-68] Zinc Chloride. In Proceedings of the 13th International Workshop on Targetry and Target Chemistry, Risø, Denmark, 26–28 July 2010. [Google Scholar]
- Pandey, M.K.; Engelbrecht, H.P.; Byrne, J.P.; Packard, A.B.; DeGrado, T.R. Production of 89Zr via the 89Y(p,n)89Zr reaction in aqueous solution: Effect of solution composition on in-target chemistry. Nucl. Med. Biol. 2014, 41, 309–316. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Augusto, R.D.S.; Liang, J.; Qin, Z.; Liu, J.; Liu, Z. Production Review of Accelerator-Based Medical Isotopes. Molecules 2022, 27, 5294. [Google Scholar] [CrossRef]
- Zhang, T.; Fan, M.; Wei, S.; Chen, S.; Yang, F. The present situation and the prospect of medical cyclotrons in China. Sci. China Phys. Mech. Astron. 2011, 54, 260–265. [Google Scholar] [CrossRef]
- Chernyaev, A.P.; Varzar, S. Particle Accelerators in Modern World. Phys. At. Nucl. 2014, 77, 1203–1215. [Google Scholar] [CrossRef]
- Ahmad Fadzil, M.F.; Ashhar, Z. Upgrades and regulatory aspects of [18F]Fluorodeoxyglucose ([18F]FDG) production using the FASTLab2 synthesizer. J. Radioanal. Nucl. Chem. 2021, 331, 99–110. [Google Scholar] [CrossRef]
- do Carmo, S.J.C.; Scott, P.; Alves, F. Production of radiometals liquid targets. EJNMMI Radiopharm. Chem. 2020, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, B.G.; Rijo, P.; Gonçalo, T.S.; Reis, C.P. Good manufacturing practices for medicinal products for human use. J. Pharm. Bioallied Sci. 2015, 7, 87–96. [Google Scholar] [PubMed]
- Gillings, N.; Hjelstuen, O.; Ballinger, J.; Behe, M.; Decristoforo, C.; Elsinga, P.; Ferrari, V.; Peitl, P.K.; Koziorowski, J.; Laverman, P.; et al. Guideline on current good radiopharmacy practice (cGRPP) for the small-scale preparation of radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2021, 6, 8. [Google Scholar] [CrossRef]
- Hendrikse, H.; Kiss, O.; Kunikowska, J.; Wadsak, W.; Decristoforo, C.; Patt, M. EANM position on the in-house preparation of radiopharmaceuticals. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Secretariat PICS. Guide to Good Manufacturing Practice for Medicinal Products (Pe 009-2); PICS: Geneva, Switzerland, 2004; pp. 1–143. [Google Scholar]
- Sciacca, G.; Martini, P.; Cisternino, S.; Mou, L.; Amico, J.; Esposito, J.; Gorgoni, G.; Cazzola, E. A Universal Cassette-Based System for the Dissolution of Solid Targets. Molecules 2021, 26, 6255. [Google Scholar] [CrossRef]
- Guerra Gomez, F.; Taniguchi, M.; Higuchi, H.; Uno, H.; Katayama, T.; Ueno, S.; Saito, K.; Morita, T. Production of Metallic Radionuclides at Sumitomo CYPRIS HM-10 Cyclotron Using an Automatic Irradiation and Dissolution Target System. J. Nucl. Med. 2020, 61 (Suppl. 1), 516. [Google Scholar]
- Hennrich, U.; Benešová, M. [68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals 2020, 13, 38. [Google Scholar] [CrossRef] [Green Version]
- Waldmann, C.M.; Stuparu, A.D.; van Dam, R.M.; Slavik, R. The Search for an Alternative to [68Ga]Ga-DOTA-TATE in Neuroendocrine Tumor Theranostics: Current State of 18F-labeled Somatostatin Analog Development. Theranostics 2019, 9, 1336–1347. [Google Scholar] [CrossRef] [PubMed]
- Archibald, S.J.; Allott, L. The aluminium-[18F]fluoride revolution: Simple radiochemistry with a big impact for radiolabelled biomolecules. EJNMMI Radiopharm. Chem. 2021, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Othman, M.F.; Abdul Razak, H.R.; Zakaria, Z.A.; Ahmad Saad, F.F.; Osman, M.A.; Yi, L.H.; Ashhar, Z.; Idris, J.; Abdul Hamid, M.H.N.; et al. Preparation, Optimisation, and In Vitro Evaluation of [18F]AlF-NOTA-Pamidronic Acid for Bone Imaging PET. Molecules 2022, 27, 7969. [Google Scholar] [CrossRef] [PubMed]


| Target Preparation | Nominal Proton Energy | Irradiation Parameters | EOB (GBq) | Specific Activity (GBq/μg) | Saturation Yield (GBq/μA) | EOP (GBq) | Ref. |
|---|---|---|---|---|---|---|---|
| Target material: Electroplated on a platinum disc Dimension: 7.0 mm 68Zn mass: 104.1 ± 2.7 mg | 14.5 MeV | Current: 30 μA Time: 60 min | 60.9 ± 1.8 | NR | 2.72 ± 0.08 | NR | [22] |
| Target material: Electroplated on a platinum backing Dimension: 10.0 mm 68Zn mass: 35.3 ± 2.2 mg | 14.5 MeV with 320 μm aluminium degrader foils | Current: 35 μA Time: 8.5 min | 6.3 ± 0.4 | 2530 | 1.26 ± 0.08 | 3.7 ± 0.18 | [38] |
| Target material: Electroplated water-cooled silver backing Dimension: 10.0 mm 68Zn mass: 300 mg | 13.0 MeV on target. helium-cooled aluminium foil energy degrader | Current: 80 µA Time: 120 min | >370 | NR | NR | 194 | [31] |
| Target material: Foil Dimension: 15.5 mm 68Zn mass: ~140 mg | 12.6 MeV with 500 μm aluminium energy degrader | Current: 25 μA Time: 68 min | 31 ± 1 | 1209 ± 18 | 2.48 ± 0.06 | 18 ± 2 | [34] |
| Target material: Electroplated on a silver backing Dimension: ~10.0 mm 68Zn mass: 216 ± 10 mg | 13.0 MeV with 500 μm aluminum foil degrader | Current: 80 μA Time: 102 min | 370 | NR | 7.1 | 175.2 | [39] |
| Cyclotron | Pressed Target Preparation | 68Zn Mass (mg) | Nominal Proton Energy (MeV) | Irradiation Parameter | Saturation Yield (GBq/μA) | EOB (GBq) | % Total 67Ga and 66Ga post 6 h | Ref. |
|---|---|---|---|---|---|---|---|---|
| TR-19 | 68Zn powder (ISOFLEX, San Francisco, CA, USA) Dimension: ~10.0 mm Thickness: 0.55 mm Density: 1.43 g/cm3 | 247 | 14 | Current: 30 μA Time: 30 min Proton beam energy: 17.2 MeV | 8.7 | 68.8 a | <2 | [33] |
| TR-24 | 68Zn powder (ISOFLEX, CA, USA) Dimension: ~10.0 mm Thickness: 0.40 mm Density: 3.18 g/cm3 | 100 | 12.5 | Current: 30 μA Time: 73 min b Proton beam energy: 17 MeV | 2.4 ± 0.12 | 37.5 ± 1.9 | 0.51 | [27] |
| Target Dissolution | Separation of Target Material and Purification | Formulation | Time (min) | EOP (GBq) | [68Ga]GaCl3 Molarity (Volume) | EOS (GBq) | AMA (GBq/µmol) | Ref. |
|---|---|---|---|---|---|---|---|---|
| S: 7 M HNO3 V: 1–2 mL Additional: adjust pH using NH4HCO2 (2–2.5 mL, 2.5 M) to pH 2 | R: Hydroxamate (200–330 mg) C: acetonitrile (10 mL), water (20 mL), 2 M HCl (2 mL), water (20 mL) W: 0.01N HCl (50 mL) E: 0.75 N HCl (2 mL) | R: CUBCX123 C: 0.5 mL 6 N HCl, 5 mL waterL: pre dilution using 0.01 M HCL (8 mL) W: 0.01 N HCl (30 mL) E: NaCl 5 M/HCl 5.5 N (12.5 μL) | <12 | NR | 5 M NaCl/5.5 M HCl (12.5 μL) | 4.6 ± 0.1 a | 28.3 ± 6.8 | [33] |
| 10 M HCl in 5 min | R: 5 g of AG® 50WX8 resin C: 20 mL of ethanol, 15 mL of 10 M HCl W: 20 mL of 10 M HCl E: 20 mL of 4 M HCl | R: 500 mg of UTEVA resin C:10 mL of ethanol, followed by 15 mL of 4 N HCl. W: 5 mL of 4 N HCl E: 2.5 mL of 0.05 M HCl | <30 | 37.5 ± 1.9 | 0.05 N HCl (2.5 mL) | NR | 9.5 ± 1.3 | [27] |
| Target Dissolution | Separation of Target Material and Purification | Formulation | Time (min) | EOP (GBq) | [68Ga]GaCl3 Molarity (Volume) | EOS (GBq) | AMA (GBq/µmol) | Ref. |
|---|---|---|---|---|---|---|---|---|
| S: 7 M HCl V: 0.5 mL | R: TK400 resin C: 7M HCl (5 mL) W: 7 M HCl (28 mL), 0.05 M HCl (0.7 mL) E: 0.05 M HCl (3.5 mL) | NA | 32 a | 3.31 | 0.05 M HCl (3.5 mL) | 1.56 b | 7.1 | [38] |
| S: 10 M HCl V: 10 mL T: <10 min | R: 5 g of 50W-X4 C: 10 M HCl (25 mL) W: 10 M HCl (30 mL) E: 4 M HCl (12 mL) | R: 100 mg UTEVA® C: 4 M HCl (10 mL) W: 4 M HCl (10 mL) E: 0.05 M HCl (2 mL) | 10 | NR | 0.05 M HCl (2.0 mL) | NR | 6.7 ± 0.8 | [22] |
| S: 9.5 M HCl V: 2 mL T: 2 min | R: UTEVA L: Pre-dilution4 M HCl (11.5 mL) W: 10 mL of 4 M, HCl and 10 mL of 2.5 M HCl E: 2 mL water and re-distributed into 10 mL of 4 M HCl | R: UTEVA W: 20 mL of 2.5 M HCl E: 1 mL water | 23 | 18 ± 2 | Water (1 mL) | 86 ± 22 c | [34] | |
| S: 30% HCl (~90 °C) V: 2 mL | R: 250 mg ZR resin. W:15 mL of 30% HCl E: 8 mL of 1 M HCl and passed through a LN Resin | R: TK200 resin. W: Nitrogen purging E: 2.5 mL of 0.1 M HCl | 35 | 194 d | 0.1 M HCl (2.5 mL) | 72.2 | 25 e | [32] |
| Internal Volume | Degrader | Support | Ref. |
|---|---|---|---|
| 2.5 mL | 250 µm foil of niobium liquid niobium-body target | 25 µm Havar® foils for helium cooling chamber and the helium cooling | [35] |
| 2.0 mL | Dual foils of 200 µm aluminum | Havar (40 µm) separated by helium cooling. | [36] |
| 2.2 mL | 200 µm aluminum foil | 25 μm Havar foil for support and 25 μm niobium foil for chemical inertness with the target media | [37] |
| Process | Method | Result | Ref. |
|---|---|---|---|
| Target material preparation and irradiation using GE PETtrace 800 cyclotron | Target: 1.7 M [68Zn]Zn(NO3)2 in 0.2 M HNO3 Current: 46 µA Time: 32 min Nominal proton energy: 12 MeV | EOB = 4.3 ± 0.3 GBq | [35] |
| Target: 1.42 M [68Zn]Zn(NO3)2 in 1.2 M HNO3 Current: 40 µA Time: 60 min Nominal proton energy: 14 MeV | EOB = 9.85 ± 1.6 GBq (266 mCi) | [36] | |
| Target: 1.0 M [68Zn]Zn(NO3)2 in 0.2–0.3 M HNO3 Volume: 2.2 mL Current: 30 μA Time: 60 min Nominal proton energy: 14.3 MeV | EOB = 3.7 GBq (100 mCi) | [37] |
| Process | Method | Result | Ref. |
|---|---|---|---|
| Separation of target material and purification, and formulation | Platform: FastLab2 Developer, GE Healthcare, Wisconsin, USA Purification: Zr Resin washed with 0.1 M HNO3 (9 mL), elute with 2 M HCl (5 mL) Formulation: TK200, elute with 0.1 N HCl (5 mL) | EOS = 2.3 ± 0.2 GBq | [35] |
| Platform: Trasis All-in-One, Belgium Purification: 100 mg, hydroxamate resin (50–100 μm); washed with of 0.005 M HNO3 (50 mL); elute with of 5.5 M HCl (7 mL). Formulation: 400 mg, AG-1X-8 anion exchange resin; elution with 2 mL of water. | NR | [36] | |
| Platform: FastLab2 Developer, GE Healthcare, Milwaukee, WI, USA Purification: Zr Resin; condition with 0.1 M HNO3 (7 mL); washed with 0.1 M HNO3 (15 mL), elution with 1.75 M HCl (5–6 mL). Formulation: TK200 resin; condition with water (7 mL) followed by 1.75 M HCl (4 mL) before use; washed with 2.0 M NaCl (3.5 mL) in 0.13 M HCl; elute with 1–2 mL H2O followed by dilute HCl to formulate | EOS= 2.0 ± 0.3 GBq 50 mCi | [37] |
| Process | Method | Result | Ref. |
|---|---|---|---|
| Radiolabeling | 100 °C for 10 min at pH 4.0–4.5 | [68Ga]Ga-PSMA-11 1.78–3.16 GBq (48.1–85.5 mCi, uncorrected) | [36] |
| 50 °C for 5 min | [68Ga]Ga-PSMA-11 were near quantitative (~1.67 GBq, 45 mCi) | [37] |
| Method | Yield and 68Zn Quality | Ref. |
|---|---|---|
| Yield: 82.6% ± 13.6 Purity: 99.5% Impurities: 0.5% Na as NaNO3 | [36] |
| Not available | [35] |
| Step | Precaution Measure |
|---|---|
| Pre-processing |
|
| Processing |
|
| Post-processing |
|
| Good Manufacturing Practice (GMP) | Good Preparation Practice (GPP) |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashhar, Z.; Ahmad Fadzil, M.F.; Othman, M.F.; Yusof, N.A.; Abdul Onny, M.A.; Mat Ail, N.; Abd Rahman, S.F. Cyclotron Production of Gallium-68 Radiopharmaceuticals Using the 68Zn(p,n)68Ga Reaction and Their Regulatory Aspects. Pharmaceutics 2023, 15, 70. https://doi.org/10.3390/pharmaceutics15010070
Ashhar Z, Ahmad Fadzil MF, Othman MF, Yusof NA, Abdul Onny MA, Mat Ail N, Abd Rahman SF. Cyclotron Production of Gallium-68 Radiopharmaceuticals Using the 68Zn(p,n)68Ga Reaction and Their Regulatory Aspects. Pharmaceutics. 2023; 15(1):70. https://doi.org/10.3390/pharmaceutics15010070
Chicago/Turabian StyleAshhar, Zarif, Muhammad Fakhrurazi Ahmad Fadzil, Muhamad Faiz Othman, Nor Azah Yusof, Muhammad Adib Abdul Onny, Noratikah Mat Ail, and Siti Fatimah Abd Rahman. 2023. "Cyclotron Production of Gallium-68 Radiopharmaceuticals Using the 68Zn(p,n)68Ga Reaction and Their Regulatory Aspects" Pharmaceutics 15, no. 1: 70. https://doi.org/10.3390/pharmaceutics15010070
APA StyleAshhar, Z., Ahmad Fadzil, M. F., Othman, M. F., Yusof, N. A., Abdul Onny, M. A., Mat Ail, N., & Abd Rahman, S. F. (2023). Cyclotron Production of Gallium-68 Radiopharmaceuticals Using the 68Zn(p,n)68Ga Reaction and Their Regulatory Aspects. Pharmaceutics, 15(1), 70. https://doi.org/10.3390/pharmaceutics15010070