Selegiline Modulates Lipid Metabolism by Activating AMPK Pathways of Epididymal White Adipose Tissues in HFD-Fed Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Glucose Tolerance Test (GTT)
2.3. Western Blotting Analysis
2.4. Hematoxylin and Eosin (H&E)
2.5. Immunohistochemistry (IHC)
2.6. Statistical Analysis
3. Results
3.1. Selegiline Prevented Obesity in HFD-Fed Mice
3.2. Selegiline Reduced Body Weight and Fat Accumulation in HFD-Fed Obese Mice
3.3. Selegiline Administration Reduced Epididymal Adipocyte Size
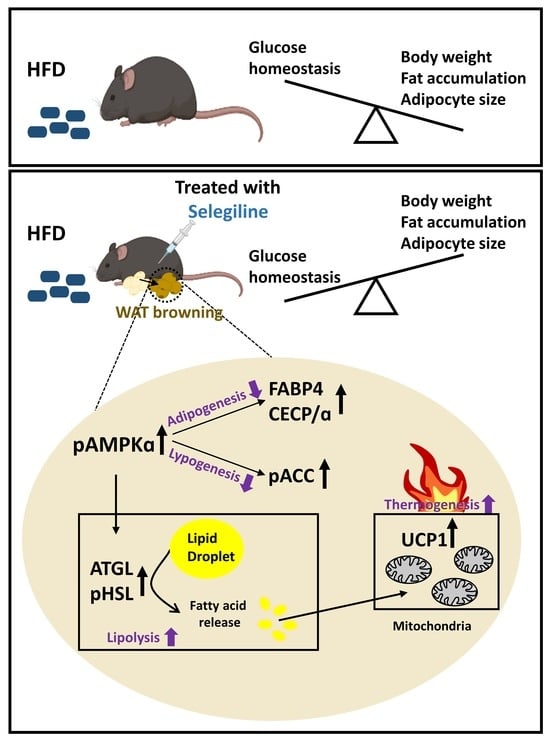
3.4. Selegiline Regulates Lipid Metabolism through an Activated AMPK Signaling Pathway in eWAT
3.5. Selegiline Induced eWAT Browning of HFD-Fed Obese Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, D.Y.; Suh, N.; Jung, M.H. Tanshinone 1 prevents high fat diet-induced obesity through activation of brown adipocytes and induction of browning in white adipocytes. Life Sci. 2022, 298, 120488. [Google Scholar] [CrossRef]
- Khafagy, R.; Dash, S. Obesity and Cardiovascular Disease: The Emerging Role of Inflammation. Front. Cardiovasc. Med. 2021, 8, 768119. [Google Scholar] [CrossRef]
- Zhang, F.; Ai, W.; Hu, X.; Meng, Y.; Yuan, C.; Su, H.; Wang, L.; Zhu, X.; Gao, P.; Shu, G.; et al. Phytol stimulates the browning of white adipocytes through the activation of AMP-activated protein kinase (AMPK) alpha in mice fed high-fat diet. Food Funct. 2018, 9, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.K.; Cho, H.W.; Song, S.E.; Im, S.S.; Bae, J.H.; Song, D.K. Oxidative stress resulting from the removal of endogenous catalase induces obesity by promoting hyperplasia and hypertrophy of white adipocytes. Redox Biol. 2020, 37, 101749. [Google Scholar] [CrossRef]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Jakab, J.; Miskic, B.; Miksic, S.; Juranic, B.; Cosic, V.; Schwarz, D.; Vcev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Seale, P.; Conroe, H.M.; Estall, J.; Kajimura, S.; Frontini, A.; Ishibashi, J.; Cohen, P.; Cinti, S.; Spiegelman, B.M. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Investig. 2011, 121, 96–105. [Google Scholar] [CrossRef]
- Pizzinat, N.; Marti, L.; Remaury, A.; Leger, F.; Langin, D.; Lafontan, M.; Carpene, C.; Parini, A. High expression of monoamine oxidases in human white adipose tissue: Evidence for their involvement in noradrenaline clearance. Biochem. Pharmacol. 1999, 58, 1735–1742. [Google Scholar] [CrossRef]
- Bour, S.; Daviaud, D.; Gres, S.; Lefort, C.; Prevot, D.; Zorzano, A.; Wabitsch, M.; Saulnier-Blache, J.S.; Valet, P.; Carpene, C. Adipogenesis-related increase of semicarbazide-sensitive amine oxidase and monoamine oxidase in human adipocytes. Biochimie 2007, 89, 916–925. [Google Scholar] [CrossRef]
- Barrand, M.A.; Callingham, B.A. Monoamine oxidase activities in brown adipose tissue of the rat: Some properties and subcellular distribution. Biochem. Pharmacol. 1982, 31, 2177–2184. [Google Scholar] [CrossRef]
- Tong, J.H.; D’Iorio, A.; Kandaswami, C. On the characteristics of mitochondrial monoamine oxidase in pancreas and adipose tissues from genetically obese mice. Can. J. Biochem. 1979, 57, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Carpene, C.; Marti, L.; Morin, N. Increased monoamine oxidase activity and imidazoline binding sites in insulin-resistant adipocytes from obese Zucker rats. World J. Biol. Chem. 2022, 13, 15–34. [Google Scholar] [CrossRef]
- Maggiorani, D.; Manzella, N.; Edmondson, D.E.; Mattevi, A.; Parini, A.; Binda, C.; Mialet-Perez, J. Monoamine Oxidases, Oxidative Stress, and Altered Mitochondrial Dynamics in Cardiac Ageing. Oxid Med. Cell Longev. 2017, 2017, 3017947. [Google Scholar] [CrossRef]
- Marti, L.; Morin, N.; Enrique-Tarancon, G.; Prevot, D.; Lafontan, M.; Testar, X.; Zorzano, A.; Carpene, C. Tyramine and vanadate synergistically stimulate glucose transport in rat adipocytes by amine oxidase-dependent generation of hydrogen peroxide. J. Pharmacol. Exp. Ther. 1998, 285, 342–349. [Google Scholar] [PubMed]
- Visentin, V.; Prevot, D.; Marti, L.; Carpene, C. Inhibition of rat fat cell lipolysis by monoamine oxidase and semicarbazide-sensitive amine oxidase substrates. Eur. J. Pharmacol. 2003, 466, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.T.; Koncsos, G.; Varga, Z.V.; Baranyai, T.; Tuza, S.; Kassai, F.; Ernyey, A.J.; Gyertyan, I.; Kiraly, K.; Olah, A.; et al. Selegiline reduces adiposity induced by high-fat, high-sucrose diet in male rats. Br. J. Pharmacol. 2018, 175, 3713–3726. [Google Scholar] [CrossRef]
- Carpene, C.; Iffiu-Soltesz, Z.; Bour, S.; Prevot, D.; Valet, P. Reduction of fat deposition by combined inhibition of monoamine oxidases and semicarbazide-sensitive amine oxidases in obese Zucker rats. Pharmacol. Res. 2007, 56, 522–530. [Google Scholar] [CrossRef]
- Carpene, C.; Abello, V.; Iffiu-Soltesz, Z.; Mercier, N.; Feve, B.; Valet, P. Limitation of adipose tissue enlargement in rats chronically treated with semicarbazide-sensitive amine oxidase and monoamine oxidase inhibitors. Pharmacol. Res. 2008, 57, 426–434. [Google Scholar] [CrossRef]
- Mattila, M.; Torsti, P. Effect of monoamine oxidase inhibitors and some related compounds on lipid metabolism in rat. Plasma free fatty acids and lipoprotein lipase of the heart and adipose tissue. Ann. Med. Exp. Biol. Fenn. 1966, 44, 397–400. [Google Scholar]
- Tian, Z.; Wang, X.; Han, T.; Sun, C. Selegiline ameliorated dyslipidemia and hepatic steatosis in high-fat diet mice. Int. Immunopharmacol. 2023, 117, 109901. [Google Scholar] [CrossRef]
- Kwon, E.; Joung, H.Y.; Liu, S.M.; Chua, S.C., Jr.; Schwartz, G.J.; Jo, Y.H. Optogenetic stimulation of the liver-projecting melanocortinergic pathway promotes hepatic glucose production. Nat. Commun. 2020, 11, 6295. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Kim, E.; Chun, S. Ginsenoside Compound K Induces Ros-Mediated Apoptosis and Autophagic Inhibition in Human Neuroblastoma Cells In Vitro and In Vivo. Int. J. Mol. Sci. 2019, 20, 4279. [Google Scholar] [CrossRef]
- Zaqout, S.; Becker, L.L.; Kaindl, A.M. Immunofluorescence Staining of Paraffin Sections Step by Step. Front. Neuroanat. 2020, 14, 582218. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Prieto, J.M.; Arbones-Mainar, J.M.; Valero, M.S.; Lopez, V. Bioactive properties of commercialised pomegranate (Punica granatum) juice: Antioxidant, antiproliferative and enzyme inhibiting activities. Food Funct. 2015, 6, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Park, J.; Hong, S.H.; Han, M.H.; Park, S.; Jung, H.I.; Noh, M. The opposite effect of isotype-selective monoamine oxidase inhibitors on adipogenesis in human bone marrow mesenchymal stem cells. Bioorg. Med. Chem. Lett. 2013, 23, 3273–3276. [Google Scholar] [CrossRef]
- Mercader, J.; Sabater, A.G.; Le Gonidec, S.; Decaunes, P.; Chaplin, A.; Gomez-Zorita, S.; Milagro, F.I.; Carpene, C. Oral Phenelzine Treatment Mitigates Metabolic Disturbances in Mice Fed a High-Fat Diet. J. Pharmacol. Exp. Ther. 2019, 371, 555–566. [Google Scholar] [CrossRef]
- Garin-Shkolnik, T.; Rudich, A.; Hotamisligil, G.S.; Rubinstein, M. FABP4 attenuates PPARgamma and adipogenesis and is inversely correlated with PPARgamma in adipose tissues. Diabetes 2014, 63, 900–911. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Liu, H.; Liu, M.; Jin, Z.; Yaqoob, S.; Zheng, M.; Cai, D.; Liu, J.; Guo, S. Ginsenoside Rg2 inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat-diet-induced obese mice through the AMPK pathway. Food Funct. 2019, 10, 3603–3614. [Google Scholar] [CrossRef]
- Long, Y.C.; Zierath, J.R. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Investig. 2006, 116, 1776–1783. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. Sensing of energy and nutrients by AMP-activated protein kinase. Am. J. Clin. Nutr. 2011, 93, 891S–896S. [Google Scholar] [CrossRef]
- Zhou, G.; Sebhat, I.K.; Zhang, B.B. AMPK activators--potential therapeutics for metabolic and other diseases. Acta Physiol. 2009, 196, 175–190. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Jarzyna, R. [AMP-activated protein kinase—The key role in metabolic regulation]. Postepy Biochem. 2006, 52, 283–288. [Google Scholar] [PubMed]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef]
- Gauthier, M.S.; Miyoshi, H.; Souza, S.C.; Cacicedo, J.M.; Saha, A.K.; Greenberg, A.S.; Ruderman, N.B. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: Potential mechanism and physiological relevance. J. Biol. Chem. 2008, 283, 16514–16524. [Google Scholar] [CrossRef]
- Foretz, M.; Taleux, N.; Guigas, B.; Horman, S.; Beauloye, C.; Andreelli, F.; Bertrand, L.; Viollet, B. Regulation of energy metabolism by AMPK: A novel therapeutic approach for the treatment of metabolic and cardiovascular diseases. Med. Sci. 2006, 22, 381–388. [Google Scholar]
- Galic, S.; Loh, K.; Murray-Segal, L.; Steinberg, G.R.; Andrews, Z.B.; Kemp, B.E. AMPK signaling to acetyl-CoA carboxylase is required for fasting- and cold-induced appetite but not thermogenesis. eLife 2018, 7, e32656. [Google Scholar] [CrossRef]
- Jang, H.M.; Han, S.K.; Kim, J.K.; Oh, S.J.; Jang, H.B.; Kim, D.H. Lactobacillus sakei Alleviates High-Fat-Diet-Induced Obesity and Anxiety in Mice by Inducing AMPK Activation and SIRT1 Expression and Inhibiting Gut Microbiota-Mediated NF-kappaB Activation. Mol. Nutr. Food Res. 2019, 63, e1800978. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Leiria, L.O.; Tseng, Y.H. Lipidomics of brown and white adipose tissue: Implications for energy metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158788. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, R.B. The role of AMP-activated protein kinase in regulating white adipose tissue metabolism. Mol. Cell Endocrinol. 2013, 366, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Yoo, H.J. The Role of Adipose Tissue Lipolysis in Diet-Induced Obesity: Focus on Vimentin. Diabetes Metab. J. 2021, 45, 43–45. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhaes, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef]
- de Pinho, L.; Andrade, J.M.; Paraiso, A.; Filho, A.B.; Feltenberger, J.D.; Guimaraes, A.L.; de Paula, A.M.; Caldeira, A.P.; de Carvalho Botelho, A.C.; Campagnole-Santos, M.J.; et al. Diet composition modulates expression of sirtuins and renin-angiotensin system components in adipose tissue. Obesity 2013, 21, 1830–1835. [Google Scholar] [CrossRef]
- Kajimura, S.; Seale, P.; Kubota, K.; Lunsford, E.; Frangioni, J.V.; Gygi, S.P.; Spiegelman, B.M. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 2009, 460, 1154–1158. [Google Scholar] [CrossRef]
- Hondares, E.; Rosell, M.; Diaz-Delfin, J.; Olmos, Y.; Monsalve, M.; Iglesias, R.; Villarroya, F.; Giralt, M. Peroxisome proliferator-activated receptor alpha (PPARalpha) induces PPARgamma coactivator 1alpha (PGC-1alpha) gene expression and contributes to thermogenic activation of brown fat: Involvement of PRDM16. J. Biol. Chem. 2011, 286, 43112–43122. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scime, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef]
- Kitaichi, Y.; Inoue, T.; Mitsui, N.; Nakagawa, S.; Kameyama, R.; Hayashishita, Y.; Shiga, T.; Kusumi, I.; Koyama, T. Selegiline remarkably improved stage 5 treatment-resistant major depressive disorder: A case report. Neuropsychiatr. Dis. Treat. 2013, 9, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Sa, M.; Yoo, E.S.; Koh, W.; Park, M.G.; Jang, H.J.; Yang, Y.R.; Bhalla, M.; Lee, J.H.; Lim, J.; Won, W.; et al. Hypothalamic GABRA5-positive neurons control obesity via astrocytic GABA. Nat. Metab. 2023, 5, 1506–1525. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joung, H.-Y.; Oh, J.-M.; Song, M.-S.; Kwon, Y.-B.; Chun, S. Selegiline Modulates Lipid Metabolism by Activating AMPK Pathways of Epididymal White Adipose Tissues in HFD-Fed Obese Mice. Pharmaceutics 2023, 15, 2539. https://doi.org/10.3390/pharmaceutics15112539
Joung H-Y, Oh J-M, Song M-S, Kwon Y-B, Chun S. Selegiline Modulates Lipid Metabolism by Activating AMPK Pathways of Epididymal White Adipose Tissues in HFD-Fed Obese Mice. Pharmaceutics. 2023; 15(11):2539. https://doi.org/10.3390/pharmaceutics15112539
Chicago/Turabian StyleJoung, Hye-Young, Jung-Mi Oh, Min-Suk Song, Young-Bae Kwon, and Sungkun Chun. 2023. "Selegiline Modulates Lipid Metabolism by Activating AMPK Pathways of Epididymal White Adipose Tissues in HFD-Fed Obese Mice" Pharmaceutics 15, no. 11: 2539. https://doi.org/10.3390/pharmaceutics15112539
APA StyleJoung, H. -Y., Oh, J. -M., Song, M. -S., Kwon, Y. -B., & Chun, S. (2023). Selegiline Modulates Lipid Metabolism by Activating AMPK Pathways of Epididymal White Adipose Tissues in HFD-Fed Obese Mice. Pharmaceutics, 15(11), 2539. https://doi.org/10.3390/pharmaceutics15112539