Cysteamine Eye Drops in Hyaluronic Acid Packaged in Innovative Single-Dose Systems, Part II: Long-Term Stability and Clinical Ocular Biopermanence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
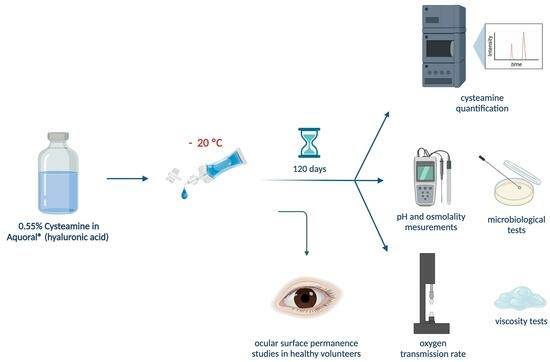
2.2.1. Elaboration and Packaging of Cysteamine Sterile Solutions
2.2.2. Long-Term Stability Study
2.2.3. Cysteamine Quantification
2.2.4. Determination of pH and Osmolality
2.2.5. Viscosity Tests
2.2.6. Microbiological Stability
2.2.7. Single-Dose Oxygen Transmission Rate
2.2.8. Ocular Surface Permanence Study in Healthy Volunteers
2.3. Statistical Analysis
3. Results
3.1. Physicochemical Stability
3.2. Microbiological Stability
3.3. Single-Dose Oxygen Transmission Rate
3.4. Ocular Surface Permanence Studies in Healthy Volunteers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, H.; Labbé, A.; Baudouin, C.; Plisson, C.; Giordano, V. Long-Term Follow-up of Cystinosis Patients Treated with 0.55% Cysteamine Hydrochloride. Br. J. Ophthalmol. 2020, 105, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Orphanet: Cystinosis. Available online: https://www.orpha.net/consor/cgi-bin/Disease_Search.php?lng=ES&data_id=11&Disease_Disease_Search_diseaseGroup=cistinosis&Disease_Disease_Search_diseaseType=Pat&Enfermedad(es)/grupo%20de%20enfermedades=Cistinosis&title=Cistinosis&search=Disease_Search_Simple (accessed on 8 August 2023).
- Cherqui, S.; Kalatzis, V.; Trugnan, G.; Antignac, C. The Targeting of Cystinosin to the Lysosomal Membrane Requires a Tyrosine-Based Signal and a Novel Sorting Motif. J. Biol. Chem. 2001, 276, 13314–13321. [Google Scholar] [CrossRef] [PubMed]
- Bäumner, S.; Weber, L.T. Nephropathic Cystinosis: Symptoms, Treatment, and Perspectives of a Systemic Disease. Front. Pediatr. 2018, 6, 58. [Google Scholar] [CrossRef]
- Andrzejewska, Z.; Nevo, N.; Thomas, L.; Chhuon, C.; Bailleux, A.; Chauvet, V.; Courtoy, P.J.; Chol, M.; Guerrera, I.C.; Antignac, C. Cystinosin Is a Component of the Vacuolar H+-ATPase-Ragulator-Rag Complex Controlling Mammalian Target of Rapamycin Complex 1 Signaling. J. Am. Soc. Nephrol. 2016, 27, 1678–1688. [Google Scholar] [CrossRef] [PubMed]
- Pisoni, R.L.; Thoene, J.G.; Christensen, H.N. Detection and Characterization of Carrier-Mediated Cationic Amino Acid Transport in Lysosomes of Normal and Cystinotic Human Fibroblasts. Role in Therapeutic Cystine Removal? J. Biol. Chem. 1985, 260, 4791–4798. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Washington, M.A.; Resnick, J.L.; Nischal, K.K.; Fedorchak, M.V. A Sustained Release Cysteamine Microsphere/Thermoresponsive Gel Eyedrop for Corneal Cystinosis Improves Drug Stability. Drug Deliv. Transl. Res. 2021, 11, 2224–2238. [Google Scholar] [CrossRef] [PubMed]
- Atallah, C.; Charcosset, C.; Greige-Gerges, H. Challenges for Cysteamine Stabilization, Quantification, and Biological Effects Improvement. J. Pharm. Anal. 2020, 10, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Iwata, F.; Kuehl, E.M.; Reed, G.F.; McCain, L.M.; Gahl, W.A.; Kaiser-Kupfer, M.I. A Randomized Clinical Trial of Topical Cysteamine Disulfide (Cystamine) versus Free Thiol (Cysteamine) in the Treatment of Corneal Cystine Crystals in Cystinosis. Mol. Genet. Metab. 1998, 64, 237–242. [Google Scholar] [CrossRef]
- Gahl, W.A.; Kuehl, E.M.; Iwata, F.; Lindblad, A.; Kaiser-Kupfer, M.I. Corneal Crystals in Nephropathic Cystinosis: Natural History and Treatment with Cysteamine Eyedrops. Mol. Genet. Metab. 2000, 71, 100–120. [Google Scholar] [CrossRef]
- Schneider, J.A. Approval of Cysteamine for Patients with Cystinosis. Pediatr. Nephrol. 1995, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Cystagon®. Prescribing Information. 1997. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/cystagon (accessed on 10 August 2023).
- Food and Drug Administration. Cystaran®. Prescribing Information. 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/200740s000lbl.pdf (accessed on 10 August 2023).
- Fernández-Ferreiro, A.; Luaces-Rodríguez, A.; Díaz-Tomé, V.; Gil-Martínez, M.; Ares, M.T.R.; Peralba, R.T.; Blanco-Méndez, J.; González-Barcia, M.; Otero-Espinar, F.J.; Lamas, M.J. Cysteamine ophthalmic hydrogel for the treatment of ocular cystinosis. Farm. Hosp. 2017, 41, 678–687. [Google Scholar] [CrossRef]
- Martín-Sabroso, C.; Alonso-González, M.; Fernández-Carballido, A.; Aparicio-Blanco, J.; Córdoba-Díaz, D.; Navarro-García, F.; Córdoba-Díaz, M.; Torres-Suárez, A.I. Limitations and Challenges in the Stability of Cysteamine Eye Drop Compounded Formulations. Pharmaceuticals 2022, 15, 2. [Google Scholar] [CrossRef]
- Reda, A.; Van Schepdael, A.; Adams, E.; Paul, P.; Devolder, D.; Elmonem, M.A.; Veys, K.; Casteels, I.; van den Heuvel, L.; Levtchenko, E. Effect of Storage Conditions on Stability of Ophthalmological Compounded Cysteamine Eye Drops. JIMD Rep. 2017, 42, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Purkiss, R. Stability of Cysteamine Hydrochloride in Solution. J. Clin. Pharm. Ther. 1977, 2, 199–203. [Google Scholar] [CrossRef]
- Brodrick, A.; Broughton, H.M.; Oakley, R.M. The Stability of an Oral Liquid Formulation of Cysteamine. J. Clin. Hosp. Pharm. 1981, 6, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.; Powell, K.; Chauhan, A. Novel Approaches for Improving Stability of Cysteamine Formulations. Int. J. Pharm. 2018, 549, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent Advances in Ocular Drug Delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Cystadrops®. Prescribing Information. 2016. Available online: https://www.ema.europa.eu/en/documents/product-information/cystadrops-epar-product-information_es.pdf (accessed on 10 August 2023).
- Food and Drug Administration. Cystadrops®. Prescribing Information. 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211302s000lbl.pdf (accessed on 10 August 2023).
- Food and Drug Administration. FDA Drug Approval Package: Cystadrops. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/211302Orig1s000TOC.cfm (accessed on 10 August 2023).
- Chatterjee, B.; Amalina, N.; Sengupta, P.; Mandal, U.K. Mucoadhesive Polymers and Their Mode of Action: A Recent Update. J. Appl. Pharm. Sci. 2017, 7, 195–203. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Castro-Balado, A.; Bandín-Vilar, E.; Cuartero-Martínez, A.; García-Quintanilla, L.; Hermelo-Vidal, G.; García-Otero, X.; Rodríguez-Martínez, L.; Mateos, J.; Hernández-Blanco, M.; Aguiar, P.; et al. Cysteamine Eye Drops in Hyaluronic Acid Packaged in Innovative Single-Dose Systems: Stability and Ocular Biopermanence. Pharmaceutics 2022, 14, 2194. [Google Scholar] [CrossRef]
- Biomed Device—Care in a Revolutionary Way COL®. Eye Drops System. Available online: http://col-eyedrops.com/index.php/it/eye-drops-system/col (accessed on 5 August 2022).
- Biomed Device—Care in a Revolutionary Way COL® Video—Kit for the Preparation of Aliquots of Eye Drops with Hemocomponents. Available online: https://www.youtube.com/watch?v=FCorvJXkUZw (accessed on 5 August 2022).
- Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. Available online: https://www.astm.org/d3985-17.html (accessed on 8 September 2023).
- Tian, L.; Qu, J.-H.; Zhang, X.-Y.; Sun, X.-G. Repeatability and Reproducibility of Noninvasive Keratograph 5M Measurements in Patients with Dry Eye Disease. J. Ophthalmol. 2016, 2016, 8013621. [Google Scholar] [CrossRef]
- Best, N.; Drury, L.; Wolffsohn, J.S. Clinical Evaluation of the Oculus Keratograph. Cont. Lens Anterior Eye 2012, 35, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Pena-Verdeal, H.; Garcia-Resua, C.; Garcia-Queiruga, J.; Sabucedo-Villamarin, B.; Yebra-Pimentel, E.; Giraldez, M.J. Diurnal Variations of Tear Film Osmolarity on the Ocular Surface. Clin. Exp. Optom. 2023, 106, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, C.; Zhu, D.; Shen, M.; Cui, L.; Wang, J. Daytime Variations of Tear Osmolarity and Tear Meniscus Volume. Eye Contact Lens 2012, 38, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Pena-Verdeal, H.; García-Resúa, C.; Ramos, L.; Yebra-Pimentel, E.; Giráldez, M.J. Diurnal Variations in Tear Film Break-up Time Determined in Healthy Subjects by Software-Assisted Interpretation of Tear Film Video Recordings. Clin. Exp. Optom. 2016, 99, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Lund, W. The Pharmaceutical CODEX: Principles & Practice of Pharmaceutics; 12e (HB); CBS Publishers & Distributors: New Delhi, India, 2009; ISBN 978-81-239-1650-7. [Google Scholar]
- Fernández Ferreiro, A. Formulación Magistral Oftálmica Antiinfecciosa; SEFH, Sociedad Española de Farmacia Hospitalaria: Madrid, Spain, 2019; ISBN 978-84-09-10764-3. [Google Scholar]
- Dutescu, R.M.; Panfil, C.; Schrage, N. Osmolarity of Prevalent Eye Drops, Side Effects, and Therapeutic Approaches. Cornea 2015, 34, 560–566. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Davies, L.N.; Dunne, M.C.M.; Gilmartin, B. Statistical Guidelines for Clinical Studies of Human Vision. Ophthalmic Physiol. Opt. 2011, 31, 123–136. [Google Scholar] [CrossRef]
- Gahl, W.A.; Thoene, J.G.; Schneider, J.A. Cystinosis. N. Engl. J. Med. 2002, 347, 111–121. [Google Scholar] [CrossRef]
- McKenzie, B.; Kay, G.; Matthews, K.H.; Knott, R.; Cairns, D. Preformulation of Cysteamine Gels for Treatment of the Ophthalmic Complications in Cystinosis. Int. J. Pharm. 2016, 515, 575–582. [Google Scholar] [CrossRef]
- Bozdag, S.; Gumus, K.; Gumus, O.; Unlu, N. Formulation and in Vitro Evaluation of Cysteamine Hydrochloride Viscous Solutions for the Treatment of Corneal Cystinosis. Eur. J. Pharm. Biopharm. 2008, 70, 260–269. [Google Scholar] [CrossRef]
- Pescina, S.; Carra, F.; Padula, C.; Santi, P.; Nicoli, S. Effect of PH and Penetration Enhancers on Cysteamine Stability and Trans-Corneal Transport. Eur. J. Pharm. Biopharm. 2016, 107, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Castro-Balado, A.; González-López, J.; Blanco-Mendez, J. Requerimientos Básicos Para La Elaboración de Colirios. In Formulación Magistral Oftálmica Antiinfecciosa; SEFH, Sociedad Española de Farmacia Hospitalaria: Madrid, Spain, 2019; pp. 63–70. ISBN 978-84-09-10764-3. [Google Scholar]
- Esteve Aquoral®. Available online: https://aquoral.es/aquoral-lagrimas-artificiales-alivio-rapido-y-duradero/aquoral/ (accessed on 5 August 2023).
- Casey-Power, S.; Ryan, R.; Behl, G.; McLoughlin, P.; Byrne, M.E.; Fitzhenry, L. Hyaluronic Acid: Its Versatile Use in Ocular Drug Delivery with a Specific Focus on Hyaluronic Acid-Based Polyelectrolyte Complexes. Pharmaceutics 2022, 14, 1479. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Song, J.S.; Choi, C.Y.; Yoon, K.C.; Lee, H.K.; Kim, H.S. A Randomized Multicenter Study Comparing 0.1%, 0.15%, and 0.3% Sodium Hyaluronate with 0.05% Cyclosporine in the Treatment of Dry Eye. J. Ocul. Pharmacol. Ther. 2017, 33, 66–72. [Google Scholar] [CrossRef]
- Karaca, E.E.; Özek, D.; Evren Kemer, Ö. Comparison Study of Two Different Topical Lubricants on Tear Meniscus and Tear Osmolarity in Dry Eye. Contact Lens Anterior Eye 2020, 43, 373–377. [Google Scholar] [CrossRef]
- You, I.C.; Li, Y.; Jin, R.; Ahn, M.; Choi, W.; Yoon, K.C. Comparison of 0.1%, 0.18%, and 0.3% Hyaluronic Acid Eye Drops in the Treatment of Experimental Dry Eye. J. Ocul. Pharmacol. Ther. 2018, 34, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.E.; Murphy, P.J.; Boulton, M. Effectiveness of Sodium Hyaluronate Eyedrops in the Treatment of Dry Eye. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Huerta Ángeles, G.; Nešporová, K. Hyaluronan and Its Derivatives for Ophthalmology: Recent Advances and Future Perspectives. Carbohydr. Polym. 2021, 259, 117697. [Google Scholar] [CrossRef]
- Ntonti, P.; Panagiotopoulou, E.-K.; Karastatiras, G.; Breyannis, N.; Tsironi, S.; Labiris, G. Impact of 0.1% Sodium Hyaluronate and 0.2% Sodium Hyaluronate Artificial Tears on Postoperative Discomfort Following Cataract Extraction Surgery: A Comparative Study. Eye Vis. 2019, 6, 6. [Google Scholar] [CrossRef]
- Biswas, S.; Sornalingam, K. The Ocular Status of Cystinosis Patients Receiving a Hospital Pharmacy-Made Preparation of Cysteamine Eye Drops: A Case Series. Ophthalmol. Ther. 2019, 8, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Labbé, A.; Le Mouhaër, J.; Plisson, C.; Baudouin, C. A New Viscous Cysteamine Eye Drops Treatment for Ophthalmic Cystinosis: An Open-Label Randomized Comparative Phase III Pivotal Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2275. [Google Scholar] [CrossRef]
- Phan, C.-M.; Ross, M.; Fahmy, K.; McEwen, B.; Hofmann, I.; Chan, V.W.Y.; Clark-Baba, C.; Jones, L. Evaluating Viscosity and Tear Breakup Time of Contemporary Commercial Ocular Lubricants on an In Vitro Eye Model. Transl. Vis. Sci. Technol. 2023, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Simmons, P.A.; Liu, H.; Carlisle-Wilcox, C.; Vehige, J.G. Efficacy and Safety of Two New Formulations of Artificial Tears in Subjects with Dry Eye Disease: A 3-Month, Multicenter, Active-Controlled, Randomized Trial. Clin. Ophthalmol. 2015, 9, 665–675. [Google Scholar] [CrossRef]
- Sergio, P.; Giancarlo, I.; Matteo, F.; Maria, D.S.C.; Gianni, P.; Parrilla, R.; Valente, P.; Luca, B. Analysis of Tear Film in Cystinosis Patients Treated with Topical Viscous Cysteamine Hydrochloride (Cystadrops®). Eur. J. Ophthalmol. 2022, 32, 3358–3362. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, G.; Pastrana, C.; Serramito, M.; Rodriguez-Pomar, C. Evaluation of Tear Meniscus by Optical Coherence Tomography after Different Sodium Hyaluronate Eyedrops Instillation. Acta Ophthalmol. 2019, 97, e162–e169. [Google Scholar] [CrossRef] [PubMed]
- Akiyama-Fukuda, R.; Usui, T.; Yoshida, T.; Yamagami, S. Evaluation of Tear Meniscus Dynamics Using Anterior Segment Swept-Source Optical Coherence Tomography After Topical Solution Instillation for Dry Eye. Cornea 2016, 35, 654–658. [Google Scholar] [CrossRef] [PubMed]







| DAY 0 | DAY 30 | DAY 60 | DAY 90 | DAY 120 | |
|---|---|---|---|---|---|
| Cysteamine quantification |  |  |  |  |  |
| pH and osmolality |  |  |  |  |  |
| Viscosity |  |  |  | ||
| Microbiology |  |  |  | ||
| Oxygen permeability |  |  |  | ||
| TMH and NIKBUT |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Balado, A.; Cuartero-Martínez, A.; Pena-Verdeal, H.; Hermelo-Vidal, G.; Schmidt, A.; Montero, B.; Hernández-Blanco, M.; Zarra-Ferro, I.; González-Barcia, M.; Mondelo-García, C.; et al. Cysteamine Eye Drops in Hyaluronic Acid Packaged in Innovative Single-Dose Systems, Part II: Long-Term Stability and Clinical Ocular Biopermanence. Pharmaceutics 2023, 15, 2589. https://doi.org/10.3390/pharmaceutics15112589
Castro-Balado A, Cuartero-Martínez A, Pena-Verdeal H, Hermelo-Vidal G, Schmidt A, Montero B, Hernández-Blanco M, Zarra-Ferro I, González-Barcia M, Mondelo-García C, et al. Cysteamine Eye Drops in Hyaluronic Acid Packaged in Innovative Single-Dose Systems, Part II: Long-Term Stability and Clinical Ocular Biopermanence. Pharmaceutics. 2023; 15(11):2589. https://doi.org/10.3390/pharmaceutics15112589
Chicago/Turabian StyleCastro-Balado, Ana, Andrea Cuartero-Martínez, Hugo Pena-Verdeal, Gonzalo Hermelo-Vidal, Anja Schmidt, Belén Montero, Manuela Hernández-Blanco, Irene Zarra-Ferro, Miguel González-Barcia, Cristina Mondelo-García, and et al. 2023. "Cysteamine Eye Drops in Hyaluronic Acid Packaged in Innovative Single-Dose Systems, Part II: Long-Term Stability and Clinical Ocular Biopermanence" Pharmaceutics 15, no. 11: 2589. https://doi.org/10.3390/pharmaceutics15112589
APA StyleCastro-Balado, A., Cuartero-Martínez, A., Pena-Verdeal, H., Hermelo-Vidal, G., Schmidt, A., Montero, B., Hernández-Blanco, M., Zarra-Ferro, I., González-Barcia, M., Mondelo-García, C., Giráldez, M. J., Yebra-Pimentel, E., Otero-Espinar, F. J., & Fernández-Ferreiro, A. (2023). Cysteamine Eye Drops in Hyaluronic Acid Packaged in Innovative Single-Dose Systems, Part II: Long-Term Stability and Clinical Ocular Biopermanence. Pharmaceutics, 15(11), 2589. https://doi.org/10.3390/pharmaceutics15112589