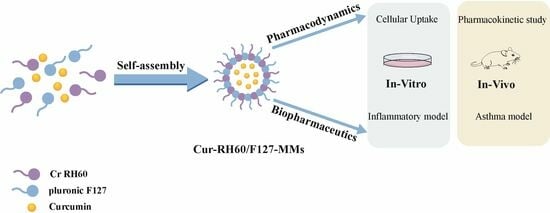
Curcumin-Loaded RH60/F127 Mixed Micelles: Characterization, Biopharmaceutical Characters and Anti-Inflammatory Modulation of Airway Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Cell Culture
2.3. Preparation of Cur-RH60/F127-MMs
2.4. Particle and Zeta Potential Analysis, Tyndall Effect, and Micromorphological Observation of Cur-RH60/F127-MMs
2.5. Determination of Encapsulation Yield (EY) and Drug Loading (DL) Capacity
2.6. Determination of Blank MMs Critical Micelle Concentration (CMC)
2.7. In Vitro Release
2.8. Intracellular Uptake and Trans-Cellular Membrane Transport
2.9. Pharmacokinetic Study
2.10. In Vitro Antioxidant Research
2.11. Effect of Cur-RH60/F127-MMs on RAW264.7 Cells Stimulated by LPS
2.12. Effects of Cur-RH60/F127-MMs on Airway Inflammation and Cytokines in OVA-Induced BALB/c Mice Asthma Model
2.13. Cell Count and IgE Level
2.14. Histopathology of the Lungs and Cytokine Levels in Bronchoalveolar Lavage Fluid (BALF)
2.15. Determination of SOD Activity, MDA Concentration, and Intercellular Adhesion Factor ICMA-1 in Lung Tissues
2.16. Data Analysis
3. Results and Discussions
3.1. Characterization of Cur-RH60/F127-MMs
3.2. Determination of Blank MMs CMC
3.3. In Vitro Curcumin Release Profile
3.4. Intracellular Uptake and Trans-Cellular Membrane Transport Study
3.5. Pharmacokinetic Study
3.6. In Vitro Antioxidant Research
3.7. Effect of Cur-RH60/F127-MMs on RAW264.7 Cells Stimulated by LPS
3.8. Effects of Cur-RH60/F127-MMs on Airway Inflammation and Cytokines in OVA-Induced BALB/c Mice Asthma Model
3.9. Effects of Cur-RH60/F127-MMs on Pathological Changes of Lung Tissue of OVA-Induced BALB/c Mice Bronchial Asthma Model
3.10. Effects of Cur-RH60/F127-MMs on ICAM-1, MDA, and SOD Levels in Lung Tissue of OVA-Induced BALB/c Mice Bronchial Asthma Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Wang, Y.; Liang, W. Delivery of drugs to cell membranes by encapsulation in PEG-PE micelles. J. Control. Release 2012, 160, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Kohay, H.; Sarisozen, C.; Sawant, R.; Jhaveri, A.; Torchilin, V.P.; Mishael, Y.G. PEG-PE/clay composite carriers for doxorubicin: Effect of composite structure on release, cell interaction and cytotoxicity. Acta Biomater. 2017, 55, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cao, W.; Liu, Y. Recent Advances in Polymeric Nano-sized Carrier Systems. Chem. J. Chin. Univ. Chin. 2020, 41, 909–923. [Google Scholar]
- Li, Z.; Xiong, X.; Peng, S.; Chen, X.; Liu, W.; Liu, C. Novel folated pluronic F127 modified liposomes for delivery of curcumin: Preparation, release, and cytotoxicity. J. Microencapsul. 2020, 37, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; El-Say, K.M.; Ahmed, O.A.; Aljaeid, B.M. Superiority of TPGS-loaded micelles in the brain delivery of vinpocetine via administration of thermosensitive intranasal gel. Int. J. Nanomed. 2019, 14, 5555–5567. [Google Scholar] [CrossRef] [PubMed]
- Jin, I.S.; Jo, M.J.; Park, C.W.; Chung, Y.B.; Kim, J.S.; Shin, D.H. Physicochemical, Pharmacokinetic, and Toxicity Evaluation of Soluplus((R)) Polymeric Micelles Encapsulating Fenbendazole. Pharmaceutics 2020, 12, 1000. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wu, Z.; Li, C.; Zhou, W.; Shaw, J.P.; Baguley, B.C.; Liu, J.; Zhang, W. Optimization of Weight Ratio for DSPE-PEG/TPGS Hybrid Micelles to Improve Drug Retention and Tumor Penetration. Pharm. Res. 2018, 35, 13. [Google Scholar] [CrossRef]
- Gu, Q.; Cai, Y.; Yang, H.; Shi, L. Therapeutic effect of curcumin on becteria-induced fulminant hepatitis in mice. Mod. Immunol. 2015, 35, 358–361. [Google Scholar]
- Hu, S.; Xu, Y.; Meng, L.; Huang, L.; Sun, H. Curcumin inhibits proliferation and promotes apoptosis of breast cancer cells. Exp. Ther. Med. 2018, 16, 1266–1272. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Li, Y.; Xie, Y.; Shan, H.; Chen, M.; Zhang, J.; Yang, X.; Zhang, Q.; Yang, X. Curcumin attenuates skeletal muscle mitochondrial impairment in COPD rats: PGC-1alpha/SIRT3 pathway involved. Chem. Biol. Interact. 2017, 277, 168–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Q.; Li, Y.; He, Z.; Li, Z.; Guo, T.; Wu, Z.; Feng, N. CD44 Assists the Topical Anti-Psoriatic Efficacy of Curcumin-Loaded Hyaluronan-Modified Ethosomes: A New Strategy for Clustering Drug in Inflammatory Skin. Theranostics 2019, 9, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Khayyal, M.T.; El-Hazek, R.M.; El-Sabbagh, W.A.; Frank, J.; Behnam, D.; Abdel-Tawab, M. Micellar solubilisation enhances the antiinflammatory activities of curcumin and boswellic acids in rats with adjuvant-induced arthritis. Nutrition 2018, 54, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Vecchione, R.; Coppola, C.; Di Cicco, C.; De Capua, A.; Piscopo, G.; Paciello, R.; Narciso, V.; Formisano, C.; Taglialatela-Scafati, O.; et al. Cardioprotective Effects of Nanoemulsions Loaded with Anti-Inflammatory Nutraceuticals against Doxorubicin-Induced Cardiotoxicity. Nutrients 2018, 10, 1304. [Google Scholar] [CrossRef] [PubMed]
- Güçlü, O.; Doğanlar, O.; Yüksel, V.; Doğanlar, Z.B. FOLFIRI-Mediated Toxicity in Human Aortic Smooth Muscle Cells and Possible Amelioration with Curcumin and Quercetin. Cardiovasc. Toxicol. 2020, 20, 139–154. [Google Scholar] [CrossRef]
- Hernandez, M.; Wicz, S.; Santamaria, M.H.; Corral, R.S. Curcumin exerts anti-inflammatory and vasoprotective effects through amelioration of NFAT-dependent endothelin-1 production in mice with acute Chagas cardiomyopathy. Mem. Inst. Oswaldo Cruz. 2018, 113, e180171. [Google Scholar] [CrossRef]
- Baj, T.; Seth, R. Role of Curcumin in Regulation of TNF-alpha Mediated Brain Inflammatory Responses. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 69–77. [Google Scholar] [CrossRef]
- Bansal, S.S.; Goel, M.; Aqil, F.; Vadhanam, M.V.; Gupta, R.C. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev. Res. 2011, 4, 1158–1171. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Sun, S.; Du, X.; Fu, M.; Khan, A.R.; Ji, J.; Liu, W.; Zhai, G. Galactosamine-modified PEG-PLA/TPGS micelles for the oral delivery of curcumin. Int. J. Pharm. 2021, 595, 120227. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Q.; Lin, G.; Shi, Y.; Gu, Z.; Ding, T. Combination of using prodrug-modified cationic liposome nanocomplexes and a potentiating strategy via targeted co-delivery of gemcitabine and docetaxel for CD44-overexpressed triple negative breast cancer therapy. Acta Biomater. 2017, 62, 257–272. [Google Scholar] [CrossRef]
- Dou, T.; Wang, J.; Han, C.; Shao, X.; Zhang, J.; Lu, W. Cellular uptake and transport characteristics of chitosan modified nanoparticles in Caco-2 cell monolayers. Int. J. Biol. Macromol. 2019, 138, 791–799. [Google Scholar] [CrossRef]
- Xiao, L.; Zhou, Y.; Zhang, X.; Ding, Y.; Li, Q. Transporter-Targeted Bile Acid-Camptothecin Conjugate for Improved Oral Absorption. Chem. Pharm. Bull. 2019, 67, 1082–1087. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, W.; Zhang, X.; Wang, Y.; Zhuang, W.; Li, F.; Li, Q. Cellular Uptake and Transport Characteristics of FL118 Derivatives in Caco-2 Cell Monolayers. Chem. Pharm. Bull. 2021, 69, 1054–1060. [Google Scholar] [CrossRef]
- Chen, X.L.; Liang, X.L.; Zhao, G.W.; Zeng, Q.Y.; Dong, W.; Ou, L.Q.; Zhang, H.N.; Jiang, Q.Y.; Liao, Z.G. Improvement of the bioavailability of curcumin by a supersaturatable self nanoemulsifying drug delivery system with incorporation of a hydrophilic polymer: In Vitro and In Vivo characterisation. J. Pharm. Pharmacol. 2021, 73, 641–652. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, H.; Zhang, F.; Sun, F.; Xin, M.; Li, M.; Li, J.; Wu, X. Novel self-nanomicellizing solid dispersion based on rebaudioside A: A potential nanoplatform for oral delivery of curcumin. Int. J. Nanomed. 2019, 14, 557–571. [Google Scholar] [CrossRef]
- Kong, Z.; Kuo, H.; Johnson, A.; Wu, L.; Chang, K. Curcumin-Loaded Mesoporous Silica Nanoparticles Markedly Enhanced Cytotoxicity in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 2918. [Google Scholar] [CrossRef]
- Tang, L.; Wu, J.; Yu, G.; Chu, J.; Yan, S.; Wang, H.; Dai, G.; Han, X. Effect of Yiqi Yangyin Zhuyu Recipe in Anti-inflammation Activities in vitro on LPS-induced RAW264.7 cells. Chin. J. Exp. Tradit. Med. Formulae 2019, 25, 141–148. [Google Scholar]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef]
- Chen, F.; Guo, N.; Cao, G.; Zhou, J.; Yuan, Z. Molecular Analysis of Curcumin-induced Polarization of Murine RAW264.7 Macrophages. J. Cardiovasc. Pharmacol. 2014, 63, 544–552. [Google Scholar] [CrossRef]
- Caputo, L.S.; Campos, M.; Dias, H.J.; Crotti, A.E.M.; Fajardo, J.B.; Vanelli, C.P.; Presto, Á.C.D.; Alves, M.S.; Aarestrup, F.M.; Paula, A.C.C. Copaiba oil suppresses inflammation in asthmatic lungs of BALB/c mice induced with ovalbumin. Int. Immunopharmacol. 2020, 80, 106177. [Google Scholar] [CrossRef]
- Okuda-Hanafusa, C.; Uchio, R.; Fuwa, A.; Kawasaki, K.; Muroyama, K.; Yamamoto, Y.; Murosaki, S. Turmeronol A and turmeronol B from Curcuma longa prevent inflammatory mediator production by lipopolysaccharide-stimulated RAW264.7 macrophages, partially via reduced NF-κB signaling. Food Funct. 2019, 10, 265. [Google Scholar] [CrossRef]
- Zhang, B.C.; Li, Z.; Xu, W.; Xiang, C.H.; Ma, Y.F. Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Am. J. Transl. Res. 2018, 10, 265–273. [Google Scholar]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, B.; Ma, B.; Zeng, Q.; Liu, W.; Song, F.; Yang, Y. Curcumin Attenuates Allergic Airway Inflammation of Young Mice through Inhibiting the Activation of p38 MAPK and AKT. J. Sun Yat-Sen Univ. (Med. Sci.) 2017, 38, 833–841. [Google Scholar]
- Subhashini, C.P.; Dash, D.; Paul, B.N.; Singh, R. Intranasal curcumin ameliorates airway inflammation and obstruction by regulating MAPKinase activation (p38, Erk and JNK) and prostaglandin D2 release in murine model of asthma. Int. Immunopharmacol. 2016, 31, 200–206. [Google Scholar] [CrossRef]
- Shahid, H.; Shahzad, M.; Shabbir, A.; Saghir, G. Immunomodulatory and Anti-Inflammatory Potential of Curcumin for the Treatment of Allergic Asthma: Effects on Expression Levels of Pro-inflammatory Cytokines and Aquaporins. Inflammation 2019, 42, 2037–2047. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, J.; Lu, M.; Zhang, W.Y. Preparation and characterization of simvastin poloxamer P123/F127 mixed micelles. Chin. J. Hosp. Pharm. 2021, 41, 1823–1829. [Google Scholar]
- Madan, J.R.; Dere, S.G.; Awasthi, R.; Dua, K. Efavirenz Loaded Mixed Polymeric Micelles: Formulation, Optimization, and In Vitro Characterization. Assay Drug Dev. Technol. 2021, 19, 322–334. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, L.; Wang, D.; Liang, Y.; Zhuang, Z.; Zhang, D.; Zhang, W. Optimization of preparation of curcumin-loaded TPGS/F127/P123 mixed micelles by uniform design methodology. Chin. Tradit. Herb. Drugs 2018, 49, 1556–1561. [Google Scholar]
- Zhang, L.; Zhu, W.; Yang, C.; Guo, H.; Yu, A.; Ji, J.; Gao, Y.; Sun, M.; Zhai, G. A novel folate-modified self-microemulsifying drug delivery system of curcumin for colon targeting. Int. J. Nanomed. 2012, 7, 151–162. [Google Scholar]
- Ke, Z.; Sun, Y.; Cheng, X.; Zhang, Z.; Wang, H.; Li, Z.; Jia, X. Research progress of polymer mixed micelles based on improving the efficacy of antitumor drugs. Acta Pharm. Sincia 2021, 56, 3047–3059. [Google Scholar]
- Fang, C.; Shi, B.; Pei, Y.-Y.; Hong, M.-H.; Wu, J.; Chen, H.-Z. In vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: Effect of MePEG molecular weight and particle size. Eur. J. Pharm. Sci. 2006, 27, 27–36. [Google Scholar] [CrossRef]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Bochner, B.S.; Busse, W.W. Allergy and asthma. J. Allergy Clin. Immunol. 2005, 115, 953–959. [Google Scholar] [CrossRef]
- Hart, P.H. Regulation of the inflammatory response in asthma by mast cell products. Immunol. Cell Biol. 2001, 79, 149–153. [Google Scholar] [CrossRef]
- Radermecker, C.; Sabatel, C.; Vanwinge, C.; Ruscitti, C.; Maréchal, P.; Perin, F.; Schyns, J.; Rocks, N.; Toussaint, M.; Cataldo, D.; et al. Locally instructed CXCR4(hi) neutrophils trigger environment-driven allergic asthma through the release of neutrophil extracellular traps. Nat. Immunol. 2019, 20, 1444–1455. [Google Scholar] [CrossRef]
- Dash, B.; Sun, X. Eosinophils set DNA traps in allergic asthma. Nat. Cell Biol. 2021, 23, 1057–1059. [Google Scholar] [CrossRef]
- Conde, E.; Bertrand, R.; Balbino, B.; Bonnefoy, J.; Stackowicz, J.; Caillot, N.; Colaone, F.; Hamdi, S.; Houmadi, R.; Loste, A.; et al. Dual vaccination against IL-4 and IL-13 protects against chronic allergic asthma in mice. Nat. Commun. 2021, 12, 2574. [Google Scholar] [CrossRef]
- Ballantyne, S.J.; Barlow, J.L.; Jolin, H.E.; Nath, P.; Williams, A.S.; Chung, K.F.; Sturton, G.; Wong, S.H.; McKenzie, A.N. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J. Allergy Clin. Immunol. 2007, 120, 1324–1331. [Google Scholar] [CrossRef]
- Saradna, A.; Do, D.C.; Kumar, S.; Fu, Q.-L.; Gao, P. Macrophage polarization and allergic asthma. Transl. Res. 2018, 191, 1–14. [Google Scholar] [CrossRef]
- Maruthamuthu, V.; Henry, L.J.; Ramar, M.K.; Kandasamy, R. Myxopyrum serratulum ameliorates airway inflammation in LPS-stimulated RAW264.7 macrophages and OVA-induced murine model of allergic asthma. J. Ethnopharmacol. 2020, 255, 112369. [Google Scholar] [CrossRef]
- Wu, D.; Li, S.; Liu, X.; Xu, J.; Jiang, A.; Zhang, Y.; Liu, Z.; Wang, J.; Zhou, E.; Wei, Z.; et al. Alpinetin prevents inflammatory responses in OVA-induced allergic asthma through modulating PI3K/AKT/NF-kappaB and HO-1 signaling pathways in mice. Int. Immunopharmacol. 2020, 89 Pt A, 107073. [Google Scholar] [CrossRef]
- de Las, C.M.; Corbi, A.L. Serotonin modulation of macrophage polarization: Inflammation and beyond. Adv. Exp. Med. Biol. 2014, 824, 89–115. [Google Scholar]
- Krishnamoorthy, N.; Khare, A.; Oriss, T.B.; Raundhal, M.; Morse, C.; Yarlagadda, M.; Wenzel, S.E.; Moore, M.L.; Peebles, R.S.; Ray, A.; et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat. Med. 2012, 18, 1525–1530. [Google Scholar] [CrossRef]
- Randolph, D.A.; Carruthers, C.J.L.; Szabo, S.J.; Murphy, K.M.; Chaplin, D.D. Modulation of airway inflammation by passive transfer of allergen-specific Th1 and Th2 cells in a mouse model of asthma. J. Immunol. 1999, 162, 2375–2383. [Google Scholar] [CrossRef]
- Stokes, J.R.; Casale, T.B. Characterization of asthma endotypes: Implications for therapy. Ann. Allergy Asthma. Immunol. 2016, 117, 121–125. [Google Scholar] [CrossRef]
- Jin, C.; Shelburne, C.P.; Li, G.; Potts, E.N.; Riebe, K.J.; Sempowski, G.D.; Foster, W.M.; Abraham, S.N. Particulate allergens potentiate allergic asthma in mice through sustained IgE-mediated mast cell activation. J. Clin. Investig. 2011, 121, 941–955. [Google Scholar] [CrossRef]
- Salvi, S.S.; Babu, K.S. Treatment of allergic asthma with monoclonal anti-IgE antibody. N. Engl. J. Med. 2000, 342, 1292–1293. [Google Scholar]
- Djukanović, R.; Wilson, S.J.; Kraft, M.; Jarjour, N.N.; Steel, M.; Chung, K.F.; Bao, W.; Fowler-Taylor, A.; Matthews, J.; Busse, W.W.; et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 583–593. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Malik, P.; Arora, S.K.; Mukherjee, T.K. Intercellular adhesion molecule-1 as a drug target in asthma and rhinitis. Respirology 2014, 19, 508–513. [Google Scholar] [CrossRef]
- Al-Harbi, N.O.; Nadeem, A.; Al-Harbi, M.M.; Imam, F.; Al-Shabanah, O.A.; Ahmad, S.F.; Sayed-Ahmed, M.M.; Bahashwan, S.A. Oxidative airway inflammation leads to systemic and vascular oxidative stress in a murine model of allergic asthma. Int. Immunopharmacol. 2015, 26, 237–245. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin- and Fish Oil-Loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-Induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef]
- Yan, Y.; Li, C.; Shi, Q.; Kong, Y.; Wang, T.; Li, Y. Research on expression of inflammatory factors in BALF of model rats with oxidative stress bronchial asthma and the intervention effects of Wentong Formula. Chin. J. Tradit. Chin. Med. 2018, 33, 1366–1369. [Google Scholar]











| Mathematical Model | Equation | Rsqr_adj | |
|---|---|---|---|
| Cur-API | Zero-order | F = 0.1163t + 3.1681 | 0.8103 |
| First-order | ln(100 − F) = −0.0013t + 4.5729 | 0.8163 | |
| Higuchi | F = 1.2523t1/2 + 1.0855 | 0.9383 | |
| Ritger–Peppas | F = 2.1951t0.3881 | 0.9424 | |
| Hixson–Crowell | (100 − F)1/3 = −0.0019t + 4.592 | 0.8143 | |
| Cur-RH60/F127-MMs | Zero-order | F = 0.5627t + 4.6594 | 0.8558 |
| First-order | ln(100 − F) = −0.0076t + 4.5603 | 0.8969 | |
| Higuchi | F = 5.9356t1/2 − 4.936 | 0.9506 | |
| Ritger–Peppas | F = 3.4830t0.5993 | 0.9319 | |
| Hixson–Crowell | (100 − F)1/3 = −0.0106t + 0.8835 | 0.8143 | |
| Parameters | Cur-API | Cur-RH60/F127-MMs |
|---|---|---|
| AUC0–12/(ng·h·mL−1) | 37.7 ± 17.22 | 348.32 ± 101.42 ** |
| AUC0–∞/(ng·h·mL−1) | 54.21 ± 28.6 | 353.64 ± 106.33 * |
| Tmax/h | 0.31 ± 0.13 | 0.88 ± 0.75 * |
| Cmax/(ng·mL−1) | 18.50 ± 1.81 | 199.84 ± 39.05 ** |
| T1/2/h | 5.16 ± 2.76 | 1.98 ± 0.91 |
| MRT(0–12)/h | 4.37 ± 0.88 | 2.11 ± 0.4 ** |
| CLz/F (L·h−1·kg−1) | 982.85 ± 314.28 | 181.97 ± 54.58 |
| Vz/F (L·kg−1) | 5235.15 ± 1049.01 | 495.40 ± 194.53 |
| Group | Total Cells | Monocytes | Lymphocytes | Granulocytes |
|---|---|---|---|---|
| Normal Control | 5.43 ± 0.31 | 0.13 ± 0.05 | 4.13 ± 0.17 | 1.17 ± 0.12 |
| Model Control | 35.03 ± 6.79 ** | 1.8 ± 0.45 ** | 18.93 ± 4.23 ** | 14.3 ± 2.61 ** |
| Positive Control (1.4 g·kg−1) | 11.03 ± 0.25 ## | 0.67 ± 0.09 ## | 4.1 ± 0.50 ## | 6.27 ± 0.58 ## |
| Cur-API (20 mg·kg−1) | 9.46 ± 1.44 ## | 0.26 ± 0.07 ## | 7.04 ± 1.30 ## | 2.16 ± 0.37 ## |
| MMs (20 mg·kg−1) | 7.6 ± 2.57 ## | 0.18 ± 0.04 ## | 5.15 ± 2.33 ## | 2.28 ± 0.47 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, Y.; Tang, T.; Zhao, G.; Dong, W.; Li, Q.; Liang, X. Curcumin-Loaded RH60/F127 Mixed Micelles: Characterization, Biopharmaceutical Characters and Anti-Inflammatory Modulation of Airway Inflammation. Pharmaceutics 2023, 15, 2710. https://doi.org/10.3390/pharmaceutics15122710
Wang X, Wang Y, Tang T, Zhao G, Dong W, Li Q, Liang X. Curcumin-Loaded RH60/F127 Mixed Micelles: Characterization, Biopharmaceutical Characters and Anti-Inflammatory Modulation of Airway Inflammation. Pharmaceutics. 2023; 15(12):2710. https://doi.org/10.3390/pharmaceutics15122710
Chicago/Turabian StyleWang, Xinli, Yanyan Wang, Tao Tang, Guowei Zhao, Wei Dong, Qiuxiang Li, and Xinli Liang. 2023. "Curcumin-Loaded RH60/F127 Mixed Micelles: Characterization, Biopharmaceutical Characters and Anti-Inflammatory Modulation of Airway Inflammation" Pharmaceutics 15, no. 12: 2710. https://doi.org/10.3390/pharmaceutics15122710
APA StyleWang, X., Wang, Y., Tang, T., Zhao, G., Dong, W., Li, Q., & Liang, X. (2023). Curcumin-Loaded RH60/F127 Mixed Micelles: Characterization, Biopharmaceutical Characters and Anti-Inflammatory Modulation of Airway Inflammation. Pharmaceutics, 15(12), 2710. https://doi.org/10.3390/pharmaceutics15122710