The Role of Microsphere Structures in Bottom-Up Bone Tissue Engineering
Abstract
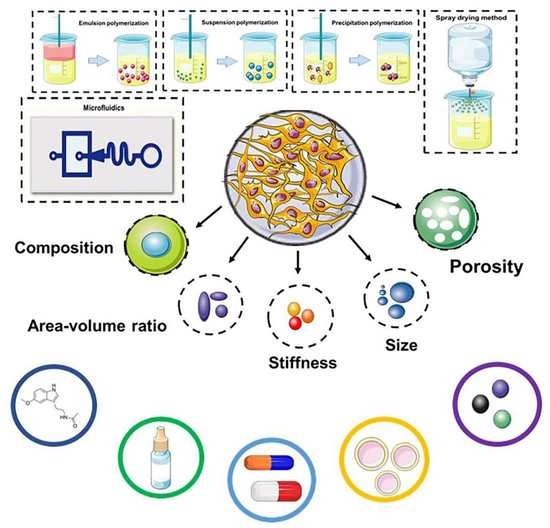
:1. Introduction
2. Fabrication of Microspheres
2.1. Emulsion Polymerization
2.2. Suspension Polymerization
2.3. Precipitation Polymerization
2.4. Spray Drying Method
2.5. Microfluidics
3. Microsphere Manufacturing Materials
3.1. Natural Materials
3.2. Synthetic Materials
3.3. Composite Materials
3.4. Auxiliary Additives
4. Application of Microspheres in Cargo Delivery
4.1. Hormones
4.2. Growth Factors
4.3. Antibiotics and Anti-Tumor Drugs
4.4. Gene Therapy and Exosomes
4.5. Metal Ions and Others
5. Novel Applications of Microspheres for Bone Tissue Engineering
5.1. 3D Culture of Seed Cells and Construction of Organoid
5.2. Endochondral Ossification for the Reparation of Large Bone Defects
5.3. Construction of Microspheres Integrating Multiple Functions
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| VEGF | vascular endothelial growth factor |
| MSCs | mesenchymal stem cells |
| KGN | kartogenin |
| CaP | calcium phosphate |
| PLA | polylactic acid |
| PGA | polyglycolic acid |
| PVA | polyvinyl alcohol |
| PLGA | polylactic acid glycolic acid copolymer |
| PCL | polycaprolactone |
| PEG | poly(ethylene glycol) |
| PEGDA | polyethylene glycol diacrylate |
| PLLA | poly(L-lactic acid) |
| TAA | triamcinolone acetonide |
| OPG | osteoprotegerin |
| TGF-β | transforming growth factor-β |
| BMP | bone morphogenetic factor |
| FGF | fibroblast growth factor |
| PDGF | platelet-derived growth factor |
| IGF | insulin-like growth factor |
| EO | endochondral osteogenesis |
| MTX | methotrexate |
| DOX | doxorubicin |
| PTX | paclitaxel |
| ETP | etoposide |
| pDNA | plasmid deoxyribonucleic acid |
| miRNA | microRNA |
| SrR | strontium ranelate |
| SB | salvianolic acid B |
| PFO | perfluorooctane |
| HPDC | human periosteum-derived cells |
| KEM | kaempferol |
| ECM | extracellular matrix |
| GelMA | gelatin methacrylate |
| EHS | electrohydrodynamic spraying |
| CS | chitosan |
| DOX | doxorubicin |
| siRNA | small interfering RNA |
| shRNA | small hairpin RNA |
References
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.E.; Martha, J.F.; Kowalski, P.; Wang, D.A.; Bode, R.; Li, L.; Kim, D.H. Prospective evaluation of chronic pain associated with posterior autologous iliac crest bone graft harvest and its effect on postoperative outcome. Health Qual. Life Outcomes 2009, 7, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapekar, M.S. Tissue engineering: Challenges and opportunities. J. Biomed. Mater. Res. 2000, 53, 617–620. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Z.; Yang, L.; Du, W.; Yu, T.; Tang, H.; Li, C.; Qiu, H. The role of natural polymers in bone tissue engineering. J. Control. Release 2021, 338, 571–582. [Google Scholar] [CrossRef]
- Xu, H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Via, A.G.; Frizziero, A.; Oliva, F. Biological properties of mesenchymal Stem Cells from different sources. Muscles Ligaments Tendons J. 2012, 2, 154–162. [Google Scholar]
- Lei, T.; Deng, S.; Chen, P.; Xiao, Z.; Cai, S.; Hang, Z.; Yang, Y.; Zhang, X.; Li, Q.; Du, H. Metformin enhances the osteogenesis and angiogenesis of human umbilical cord mesenchymal stem cells for tissue regeneration engineering. Int. J. Biochem. Cell Biol. 2021, 141, 106086. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, R.; Wang, L.; Chen, L.; Lin, Z.; Panayi, A.C.; Xue, H.; Li, H.; Xiong, L.; Liu, G. Exosomes Derived from Bone Mesenchymal Stem Cells Alleviate Compression-Induced Nucleus Pulposus Cell Apoptosis by Inhibiting Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 2310025. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tian, N.; Li, S.; Li, K.; Guo, H.; Zhang, H.; Jin, H.; An, M.; Yu, X. Extracellular vesicles secreted from mesenchymal stem cells exert anti-apoptotic and anti-inflammatory effects via transmitting microRNA-18b in rats with diabetic retinopathy. Int. Immunopharmacol. 2021, 101 Pt B, 108234. [Google Scholar] [CrossRef]
- Dixon, D.T.; Gomillion, C.T. Conductive Scaffolds for Bone Tissue Engineering: Current State and Future Outlook. J. Funct. Biomater. 2021, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, P.; Nair, A.; Dey, J.; Yang, J.; Tang, L. Method to analyze three-dimensional cell distribution and infiltration in degradable scaffolds. Tissue Eng. Part C Methods 2008, 14, 319–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuli, R.; Li, W.J.; Tuan, R.S. Current state of cartilage tissue engineering. Arthritis Res. Ther. 2003, 5, 235–238. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef]
- Leferink, A.; Schipper, D.; Arts, E.; Vrij, E.; Rivron, N.; Karperien, M.; Mittmann, K.; van Blitterswijk, C.; Moroni, L.; Truckenmüller, R. Engineered micro-objects as scaffolding elements in cellular building blocks for bottom-up tissue engineering approaches. Adv. Mater. 2014, 26, 2592–2599. [Google Scholar] [CrossRef]
- Griffin, D.R.; Weaver, W.M.; Scumpia, P.O.; Di Carlo, D.; Segura, T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015, 14, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Turgeman, G.; Pittman, D.D.; Müller, R.; Kurkalli, B.G.; Zhou, S.; Pelled, G.; Peyser, A.; Zilberman, Y.; Moutsatsos, I.K.; Gazit, D. Engineered human mesenchymal stem cells: A novel platform for skeletal cell mediated gene therapy. J. Gene Med. 2001, 3, 240–251. [Google Scholar] [CrossRef]
- Park, J.H.; Pérez, R.A.; Jin, G.Z.; Choi, S.J.; Kim, H.W.; Wall, I.B. Microcarriers designed for cell culture and tissue engineering of bone. Tissue Eng. Part B Rev. 2013, 19, 172–190. [Google Scholar] [CrossRef] [PubMed]
- Biondi, M.; Ungaro, F.; Quaglia, F.; Netti, P.A. Controlled drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Mouriño, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface 2010, 7, 209–227. [Google Scholar] [CrossRef] [Green Version]
- Tavassoli, H.; Alhosseini, S.N.; Tay, A.; Chan, P.P.Y.; Oh, S.K.W.; Warkiani, M.E. Large-scale production of stem cells utilizing microcarriers: A biomaterials engineering perspective from academic research to commercialized products. Biomaterials 2018, 181, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Mealy, J.E.; Chung, J.J.; Jeong, H.H.; Issadore, D.; Lee, D.; Atluri, P.; Burdick, J.A. Injectable Granular Hydrogels with Multifunctional Properties for Biomedical Applications. Adv. Mater. 2018, 30, e1705912. [Google Scholar] [CrossRef] [PubMed]
- Dupont, H.; Héroguez, V.; Schmitt, V. Elaboration of capsules from Pickering double emulsion polymerization stabilized solely by cellulose nanocrystals. Carbohydr. Polym. 2022, 279, 118997. [Google Scholar] [CrossRef] [PubMed]
- Honciuc, A.; Negru, O.I. Role of Surface Energy of Nanoparticle Stabilizers in the Synthesis of Microspheres via Pickering Emulsion Polymerization. Nanomaterials 2022, 12, 995. [Google Scholar] [CrossRef]
- Fan, J.B.; Song, Y.; Liu, H.; Lu, Z.; Zhang, F.; Liu, H.; Meng, J.; Gu, L.; Wang, S.; Jiang, L. A general strategy to synthesize chemically and topologically anisotropic Janus particles. Sci. Adv. 2017, 3, e1603203. [Google Scholar] [CrossRef] [Green Version]
- Yin, G.; Zheng, Z.; Wang, H.; Du, Q.; Zhang, H. Preparation of graphene oxide coated polystyrene microspheres by Pickering emulsion polymerization. J. Colloid Interface Sci. 2013, 394, 192–198. [Google Scholar] [CrossRef]
- McClements, D.J. Designing biopolymer microgels to encapsulate, protect and deliver bioactive components: Physicochemical aspects. Adv. Colloid Interface Sci. 2017, 240, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Dong, J.; Li, X.; Wang, S.; Ou, J.; Ye, M. One-step synthesis of hydrophilic microspheres for highly selective enrichment of N-linked glycopeptides. Anal. Chim. Acta 2020, 1130, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Z.; Xu, J.; Ahmad, M.; Zhang, H.; Zhang, A.; Zhang, Q.; Kou, X.; Zhang, B. Surface Microstructure Regulation of Porous Polymer Microspheres by Volume Contraction of Phase Separation Process in Traditional Suspension Polymerization System. Macromol. Rapid Commun. 2019, 40, e1800768. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Su, Y.; Zhang, H.; Liu, N.; Wang, Z.; Gao, X.; Gao, J.; Zheng, A. Poly(lactic-co-glycolic acid) microsphere production based on quality by design: A review. Drug Deliv. 2021, 28, 1342–1355. [Google Scholar] [CrossRef]
- Yin, J.; Sun, W.; Song, X.; Ji, H.; Yang, Y.; Sun, S.; Zhao, W.; Zhao, C. Precipitated droplets in-situ cross-linking polymerization towards hydrogel beads for ultrahigh removal of positively charged toxins. Sep. Purif. Technol. 2020, 238, 116497. [Google Scholar] [CrossRef]
- Zetterlund, P.B.; Thickett, S.C.; Perrier, S.; Bourgeat-Lami, E.; Lansalot, M. Controlled/Living Radical Polymerization in Dispersed Systems: An Update. Chem. Rev. 2015, 115, 9745–9800. [Google Scholar] [CrossRef]
- Liu, X.; Debije, M.G.; Heuts, J.P.; Schenning, A. Liquid-Crystalline Polymer Particles Prepared by Classical Polymerization Techniques. Chemistry 2021, 27, 14168–14178. [Google Scholar] [CrossRef]
- Mendonsa, N.; Almutairy, B.; Kallakunta, V.R.; Sarabu, S.; Thipsay, P.; Bandari, S.; Repka, M.A. Manufacturing strategies to develop amorphous solid dispersions: An overview. J. Drug Deliv. Sci. Technol. 2020, 55, 101459. [Google Scholar] [CrossRef]
- Quinlan, E.; López-Noriega, A.; Thompson, E.M.; Hibbitts, A.; Cryan, S.A.; O’Brien, F.J. Controlled release of vascular endothelial growth factor from spray-dried alginate microparticles in collagen-hydroxyapatite scaffolds for promoting vascularization and bone repair. J. Tissue Eng. Regen. Med. 2017, 11, 1097–1109. [Google Scholar] [CrossRef]
- Shakeri, A.; Khan, S.; Didar, T.F. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab Chip 2021, 21, 3053–3075. [Google Scholar] [CrossRef]
- Liu, T.; Weng, W.; Zhang, Y.; Sun, X.; Yang, H. Applications of Gelatin Methacryloyl (GelMA) Hydrogels in Microfluidic Technique-Assisted Tissue Engineering. Molecules 2020, 25, 5305. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.T.; Liu, M.; Xu, Z.R. Microfluidic fabrication of multifunctional particles and their analytical applications. Talanta 2014, 121, 163–177. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Liu, W.; Zhang, Y.; Pang, B.; Liu, H.; Zhang, Y.; Zhang, H.; Zhang, L.; Liao, H.; Ren, C.; et al. Continuous microfluidic encapsulation of single mesenchymal stem cells using alginate microgels as injectable fillers for bone regeneration. Acta Biomater. 2020, 111, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, G.; Ruan, H.; Chen, K.; Cai, Z.; Lu, G.; Li, R.; Deng, L.; Cai, M.; Cui, W. Capturing Magnesium Ions via Microfluidic Hydrogel Microspheres for Promoting Cancellous Bone Regeneration. ACS Nano 2021, 15, 13041–13054. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, Y.; Wang, F.; Deng, L.; Xu, X.; Cui, W. Microfluidic liposomes-anchored microgels as extended delivery platform for treatment of osteoarthritis. Chem. Eng. J. 2020, 400, 126004. [Google Scholar] [CrossRef]
- Ressler, A. Chitosan-Based Biomaterials for Bone Tissue Engineering Applications: A Short Review. Polymers 2022, 14, 3430. [Google Scholar] [CrossRef]
- Hu, D.; Ren, Q.; Li, Z.; Zhang, L. Chitosan-Based Biomimetically Mineralized Composite Materials in Human Hard Tissue Repair. Molecules 2020, 25, 4785. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Chitosan as a vehicle for growth factor delivery: Various preparations and their applications in bone tissue regeneration. Int. J. Biol. Macromol. 2017, 104 Pt B, 1383–1397. [Google Scholar] [CrossRef]
- Islam, N.; Dmour, I.; Taha, M.O. Degradability of chitosan micro/nanoparticles for pulmonary drug delivery. Heliyon 2019, 5, e01684. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Cui, F.; Shi, K.; Wang, J.; Niu, M.; Ma, R. The effect of chitosan molecular weight on the characteristics of spray-dried methotrexate-loaded chitosan microspheres for nasal administration. Drug Dev. Ind. Pharm. 2009, 35, 379–386. [Google Scholar] [CrossRef]
- Mathieu, M.; Vigier, S.; Labour, M.N.; Jorgensen, C.; Belamie, E.; Noel, D. Induction of mesenchymal stem cell differentiation and cartilage formation by cross-linker-free collagen microspheres. Eur. Cells Mater. 2014, 28, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Mumcuoglu, D.; Fahmy-Garcia, S.; Ridwan, Y.; Nicke, J.; Farrell, E.; Kluijtmans, S.G.; van Osch, G.J. Injectable BMP-2 delivery system based on collagen-derived microspheres and alginate induced bone formation in a time- and dose-dependent manner. Eur. Cell Mater. 2018, 35, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.J.; Song, E.H.; Park, C.; Lee, H.; Kang, I.G.; Kim, H.E.; Jeong, S.H. Porous calcium phosphate-collagen composite microspheres for effective growth factor delivery and bone tissue regeneration. Mater. Sci. Eng. C 2020, 109, 110480. [Google Scholar] [CrossRef] [PubMed]
- Link, D.P.; van den Dolder, J.; van den Beucken, J.J.; Habraken, W.; Soede, A.; Boerman, O.C.; Mikos, A.G.; Jansen, J.A. Evaluation of an orthotopically implanted calcium phosphate cement containing gelatin microparticles. J. Biomed. Mater. Res. A 2009, 90, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, X. Intraarticular treatments for osteoarthritis: New perspectives. Curr. Drug Targets 2010, 11, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Nejadnik, M.R.; Mikos, A.G.; Jansen, J.A.; Leeuwenburgh, S.C. Facilitating the mineralization of oligo(poly(ethylene glycol) fumarate) hydrogel by incorporation of hydroxyapatite nanoparticles. J. Biomed. Mater. Res. A 2012, 100, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Khanna, O.; Larson, J.C.; Moya, M.L.; Opara, E.C.; Brey, E.M. Generation of alginate microspheres for biomedical applications. J. Vis. Exp. 2012, 66, e3388. [Google Scholar] [CrossRef] [Green Version]
- Jayachandran, V.; Murugan, S.S.; Dalavi, P.A.; Vishalakshi, Y.D.G.; Seong, G.H. Alginate-based Composite Microspheres: Preparations and Applications for Bone Tissue Engineering. Curr. Pharm. Des. 2022, 28, 1067–1081. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, L.; Wu, H.; Yang, T.; Zhang, Q.; Tian, Y.; Liu, Y.; Han, X.; Guo, W.; He, M.; et al. Alginate/laponite hydrogel microspheres co-encapsulating dental pulp stem cells and VEGF for endodontic regeneration. Acta Biomater. 2020, 113, 305–316. [Google Scholar] [CrossRef]
- Van Tomme, S.R.; van Nostrum, C.F.; de Smedt, S.C.; Hennink, W.E. Degradation behavior of dextran hydrogels composed of positively and negatively charged microspheres. Biomaterials 2006, 27, 4141–4148. [Google Scholar] [CrossRef]
- Shive, M.S.; Anderson, J.M. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.D.; Son, S.; Sadiasa, A.; Min, Y.K.; Lee, B.T. Preparation and characterization of PLGA microspheres by the electrospraying method for delivering simvastatin for bone regeneration. Int. J. Pharm. 2013, 443, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, Y.; Wang, L.; Pan, J.; Xu, Y.; Li, S.; Huang, D.; Chen, J.; Liang, Z.; Yin, P.; et al. Injectable microfluidic hydrogel microspheres based on chitosan and poly(ethylene glycol) diacrylate (PEGDA) as chondrocyte carriers. RSC Adv. 2020, 10, 39662–39672. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wei, P.; Yuan, Z.; Jing, W.; Cao, J.; Li, G.; Guo, J.; Wang, H.; Chen, D.; Cai, Q. Osteoconductive and osteoinductive biodegradable microspheres serving as injectable micro-scaffolds for bone regeneration. J. Biomater. Sci. Polym. Ed. 2021, 32, 229–247. [Google Scholar] [CrossRef]
- Teng, S.H.; Liang, M.H.; Wang, P.; Luo, Y. Biomimetic composite microspheres of collagen/chitosan/nano-hydroxyapatite: In-situ synthesis and characterization. Mater. Sci. Eng. C 2016, 58, 610–613. [Google Scholar] [CrossRef]
- Boukari, Y.; Qutachi, O.; Scurr, D.J.; Morris, A.P.; Doughty, S.W.; Billa, N. A dual-application poly (dl-lactic-co-glycolic) acid (PLGA)-chitosan composite scaffold for potential use in bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 1966–1983. [Google Scholar] [CrossRef] [Green Version]
- Pei, M.; Jia, X.; Li, G.; Liu, P. Versatile Polymeric Microspheres with Tumor Microenvironment Bioreducible Degradation, pH-Activated Surface Charge Reversal, pH-Triggered “off-on” Fluorescence and Drug Release as Theranostic Nanoplatforms. Mol. Pharm. 2019, 16, 227–237. [Google Scholar] [CrossRef]
- Chen, M.; Xu, X.; Shu, G.; Lu, C.; Wu, J.; Lv, X.; Song, J.; Wu, F.; Chen, C.; Zhang, N.; et al. Multifunctional Microspheres Dual-Loaded with Doxorubicin and Sodium Bicarbonate Nanoparticles to Introduce Synergistic Trimodal Interventional Therapy. ACS Appl. Bio. Mater. 2021, 4, 3476–3489. [Google Scholar] [CrossRef]
- Li, X.; Zhi, D.; Hu, J.; Xu, S.; Liu, H.; Hu, Y. Synthesis of mesoporous silica-gel core-shell structural microparticles and their multiple drug delivery. Drug Deliv. 2015, 22, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Myers, P.; Zhang, H. Silica Microspheres-in-Pores Composite Monoliths with Fluorescence and Potential for Water Remediation. Nanomaterials 2021, 11, 2681. [Google Scholar] [CrossRef] [PubMed]
- Vishnevetskii, D.V.; Averkin, D.V.; Efimov, A.A.; Lizunova, A.A.; Ivanova, A.I.; Pakhomov, P.M.; Ruehl, E. Ag/α-Ag2MoO4/h-MoO3 nanoparticle based microspheres: Synthesis and photosensitive properties. Soft Matter 2021, 17, 10416–10420. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Shin, J.Y.; Oh, S.H.; Byun, J.H.; Lee, J.H. PCL/HA Hybrid Microspheres for Effective Osteogenic Differentiation and Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 5172–5180. [Google Scholar] [CrossRef]
- Zha, Q.; Zhang, L.; Guo, Y.; Bao, R.; Shi, F.; Shi, Y. Preparation and Study of Folate Modified Albumin Targeting Microspheres. J. Oncol. 2022, 2022, 3968403. [Google Scholar] [CrossRef]
- Tao, C.; Huang, J.; Lu, Y.; Zou, H.; He, X.; Chen, Y.; Zhong, Y. Development and characterization of GRGDSPC-modified poly(lactide-co-glycolide acid) porous microspheres incorporated with protein-loaded chitosan microspheres for bone tissue engineering. Colloids Surf. B Biointerfaces 2014, 122, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Meng, X.; Yang, W.; Zhang, J.; Sun, P.; Zhang, H.; Fang, X.; Wang, D.A.; Fan, C. Progress of gelatin-based microspheres (GMSs) as delivery vehicles of drug and cell. Mater. Sci. Eng. C 2021, 122, 111949. [Google Scholar] [CrossRef]
- Berkland, C.; King, M.; Cox, A.; Kim, K.; Pack, D.W. Precise control of PLG microsphere size provides enhanced control of drug release rate. J. Control. Release 2002, 82, 137–147. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Q.; Zhou, A.; Wang, Y.; Zhang, J.; Xiong, R.; Lenders, V.; Manshian, B.B.; Hua, D.; Soenen, S.J.; et al. Core-shell microparticles: From rational engineering to diverse applications. Adv. Colloid Interface Sci. 2022, 299, 102568. [Google Scholar] [CrossRef]
- Li, T.; Chen, T.; Chen, H.; Wang, Q.; Liu, Z.; Fang, L.; Wan, M.; Mao, C.; Shen, J. Engineered Platelet-Based Micro/Nanomotors for Cancer Therapy. Small 2021, 17, e2104912. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Liu, T.T.; Sang, Z.T.; Lin, Z.S.; Su, X.; Sun, X.T.; Yang, H.Z.; Wang, T.; Guo, S. Microfluidic Preparation of Janus Microparticles With Temperature and pH Triggered Degradation Properties. Front. Bioeng. Biotechnol. 2021, 9, 756758. [Google Scholar] [CrossRef]
- Lantigua, D.; Wu, X.; Suvarnapathaki, S.; Nguyen, M.A.; Camci-Unal, G. Composite Scaffolds from Gelatin and Bone Meal Powder for Tissue Engineering. Bioengineering 2021, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, R.; Balbinot, G.S.; Athirasala, A.; Collares, F.M.; Sereda, G.; Bertassoni, L.E. Nanoscale mineralization of cell-laden methacrylated gelatin hydrogels using calcium carbonate-calcium citrate core-shell microparticles. J. Mater. Chem. B 2021, 9, 9583–9593. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Wang, S.; Qi, Z.; Zhang, W.; Sun, Y. BMP-2-releasing gelatin microspheres/PLGA scaffolds for bone repairment of X-ray-radiated rabbit radius defects. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1662–1673. [Google Scholar] [CrossRef]
- Soran, Z.; Aydin, R.S.; Gumusderelioglu, M. Chitosan scaffolds with BMP-6 loaded alginate microspheres for periodontal tissue engineering. J. Microencapsul. 2012, 29, 770–780. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Du, Y.; Sun, J.; Bi, W.; Liu, W.; Li, R.; Wu, X.; Yang, F.; Song, L.; et al. Dual Biosignal-Functional Injectable Microspheres for Remodeling Osteogenic Microenvironment. Small 2022, 18, e2201656. [Google Scholar] [CrossRef] [PubMed]
- Jaklenec, A.; Hinckfuss, A.; Bilgen, B.; Ciombor, D.M.; Aaron, R.; Mathiowitz, E. Sequential release of bioactive IGF-I and TGF-beta 1 from PLGA microsphere-based scaffolds. Biomaterials 2008, 29, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, G.; Bai, S.; Feng, Z.; Dong, Y.; Zhou, J.; Qin, H.; Zhao, Y. MAPs/bFGF-PLGA microsphere composite-coated titanium surfaces promote increased adhesion and proliferation of fibroblasts. Biomed. Mater. 2014, 9, 035006. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wang, S.; Wu, H.; Ju, W.; Peng, J.; Qahtan, A.S.; Chen, C.; Lu, Y.; Peng, J.; Zhang, X.; et al. Optimization of release pattern of FGF-2 and BMP-2 for osteogenic differentiation of low-population density hMSCs. J. Biomed. Mater. Res. A 2015, 103, 252–261. [Google Scholar] [CrossRef]
- Zhang, B.J.; Han, Z.W.; Duan, K.; Mu, Y.D.; Weng, J. Multilayered pore-closed PLGA microsphere delivering OGP and BMP-2 in sequential release patterns for the facilitation of BMSCs osteogenic differentiation. J. Biomed. Mater. Res. A 2018, 106, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.T.; Dai, N.T.; Hsu, S.H. Biodegradable water-based polyurethane scaffolds with a sequential release function for cell-free cartilage tissue engineering. Acta Biomater. 2019, 88, 301–313. [Google Scholar] [CrossRef]
- Chong, L.Y.; Chien, L.Y.; Chung, M.C.; Liang, K.; Lim, J.C.; Fu, J.H.; Wang, C.H.; Chang, P.C. Controlling the proliferation and differentiation stages to initiate periodontal regeneration. Connect. Tissue Res. 2013, 54, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, X.; Zhao, S.; Wu, H.; Xu, H.H. Porous chitosan bilayer membrane containing TGF-beta1 loaded microspheres for pulp capping and reparative dentin formation in a dog model. Dent. Mater. 2014, 30, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Long, T.; Chen, W.; Ning, C.Q.; Zhu, Z.A.; Guo, Y.P. Bactericidal property and biocompatibility of gentamicin-loaded mesoporous carbonated hydroxyapatite microspheres. Mater. Sci. Eng. C 2013, 33, 3583–3591. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Gong, Y.; Liu, S.; Pei, Y.; Luo, X. Functionalized phosphorylated cellulose microspheres: Design, characterization and ciprofloxacin loading and releasing properties. Carbohydr. Polym. 2021, 254, 117421. [Google Scholar] [CrossRef]
- He, J.; Hu, X.; Cao, J.; Zhang, Y.; Xiao, J.; Peng, L.; Chen, D.; Xiong, C.; Zhang, L. Chitosan-coated hydroxyapatite and drug-loaded polytrimethylene carbonate/polylactic acid scaffold for enhancing bone regeneration. Carbohydr. Polym. 2021, 253, 117198. [Google Scholar] [CrossRef]
- Jin, L.; Ding, Y.; Feng, M.; Cao, Q. Preparation oral levofloxacin colon-specific microspheres delivery: In vitro and in vivo studies. Drug Deliv. 2016, 23, 992–998. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Zhou, K.; Chen, C.; Jiang, M.; Zhang, Y.; Luo, H.; Zhang, D. Hollow and porous hydroxyapatite microspheres prepared with an O/W emulsion by spray freezing method. Mater. Sci. Eng. C 2016, 69, 1068–1074. [Google Scholar] [CrossRef]
- Ding, Z.; Yuan, Q.; Huang, K.; Gu, Z.; Xuan, M.; Xu, Q.; Xu, M.; Ye, Q.; Li, L.; Xie, H. Double-Layer Microsphere Incorporated with Strontium Doped Calcium Polyphosphate Scaffold for Bone Regeneration. J. Biomed. Nanotechnol. 2019, 15, 1223–1231. [Google Scholar] [CrossRef]
- Pichayakorn, W.; Boonme, P. Evaluation of cross-linked chitosan microparticles containing metronidazole for periodontitis treatment. Mater. Sci. Eng. C 2013, 33, 1197–1202. [Google Scholar] [CrossRef]
- Wang, J.; Helder, L.; Shao, J.; Jansen, J.A.; Yang, M.; Yang, F. Encapsulation and release of doxycycline from electrospray-generated PLGA microspheres: Effect of polymer end groups. Int. J. Pharm. 2019, 564, 1–9. [Google Scholar] [CrossRef]
- Nanjo, Y.; Ishii, Y.; Kimura, S.; Fukami, T.; Mizoguchi, M.; Suzuki, T.; Tomono, K.; Akasaka, Y.; Ishii, T.; Takahashi, K.; et al. Effects of slow-releasing colistin microspheres on endotoxin-induced sepsis. J. Infect. Chemother. 2013, 19, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Muvaffak, A.; Gurhan, I.; Gunduz, U.; Hasirci, N. Preparation and characterization of a biodegradable drug targeting system for anticancer drug delivery: Microsphere-antibody conjugate. J. Drug Target. 2005, 13, 151–159. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, H.I.; Chang, W.S.; Choi, J.S. Triphenylphosphonium-conjugated glycol chitosan microspheres for mitochondria-targeted drug delivery. Int. J. Biol. Macromol. 2021, 167, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Tang, S.; Fu, Q. Pharmacokinetics and biodistribution of paclitaxel-loaded microspheres. Arzneimittelforschung 2012, 62, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tang, Y.; Zhu, Z.; Zhao, H.; Yao, J.; Sun, D. Paclitaxel and etoposide-loaded Poly (lactic-co-glycolic acid) microspheres fabricated by coaxial electrospraying for dual drug delivery. J. Biomater. Sci. Polym. Ed. 2018, 29, 1949–1963. [Google Scholar] [CrossRef]
- Pandey, G.; Yadav, S.K.; Mishra, B. Preparation and characterization of isoniazid and lamivudine co-loaded polymeric microspheres. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Yuan, Z.; Zhang, W.; Wei, D.; Hu, N. Preparation, in vitro release and antibacterial activity evaluation of rifampicin and moxifloxacin-loaded poly(D,L-lactide-co-glycolide) microspheres. Artif. Cells Nanomed. Biotechnol. 2019, 47, 790–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa-Alfaro, D.; Andres-Guerrero, V.; Fernandez-Bueno, I.; Garcia-Gutierrez, M.T.; Gil-Alegre, E.; Molina-Martinez, I.T.; Pastor-Jimeno, J.C.; Herrero-Vanrell, R.; Bravo-Osuna, I. Dexamethasone PLGA Microspheres for Sub-Tenon Administration: Influence of Sterilization and Tolerance Studies. Pharmaceutics 2021, 13, 228. [Google Scholar] [CrossRef]
- Gurler, E.B.; Ergul, N.M.; Ozbek, B.; Ekren, N.; Oktar, F.N.; Haskoylu, M.E.; Oner, E.T.; Eroglu, M.S.; Ozbeyli, D.; Korkut, V.; et al. Encapsulated melatonin in polycaprolactone (PCL) microparticles as a promising graft material. Mater. Sci. Eng. C 2019, 100, 798–808. [Google Scholar] [CrossRef]
- Gan, M.; Zhou, Q.; Ge, J.; Zhao, J.; Wang, Y.; Yan, Q.; Wu, C.; Yu, H.; Xiao, Q.; Wang, W.; et al. Precise in-situ release of microRNA from an injectable hydrogel induces bone regeneration. Acta Biomater. 2021, 135, 289–303. [Google Scholar] [CrossRef]
- Li, H.; Hu, Y.; Zeng, M.; Yang, J.; Fan, X.; Wang, Y.; Xie, J. Exosomes From Human Urine-Derived Stem Cells Encapsulated Into PLGA Nanoparticles for Therapy in Mice with Particulate Polyethylene-Induced Osteolysis. Front. Med. 2021, 8, 781449. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Li, Y.; Yang, Y.; Fang, Z.; Chen, X.; Wang, Y.; Kang, J.; Qu, X.; Yuan, W.; Dai, K.; et al. Osteoinductivity and Antibacterial Properties of Strontium Ranelate-Loaded Poly(Lactic-co-Glycolic Acid) Microspheres with Assembled Silver and Hydroxyapatite Nanoparticles. Front. Pharmacol. 2018, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, J.; Li, S.; Xu, L.; Wang, R.; Chen, R.; Sun, Y. The size-controllable preparation of chitosan/silver nanoparticle composite microsphere and its antimicrobial performance. Carbohydr. Polym. 2019, 220, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Boldbaatar, K.; Dashnyam, K.; Knowles, J.C.; Lee, H.H.; Lee, J.H.; Kim, H.W. Dual-ion delivery for synergistic angiogenesis and bactericidal capacity with silica-based microsphere. Acta Biomater. 2019, 83, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Carmo, A.; Sartoretto, S.C.; Alves, A.; Granjeiro, J.M.; Miguel, F.B.; Calasans-Maia, J.; Calasans-Maia, M.D. Alveolar bone repair with strontium-containing nanostructured carbonated hydroxyapatite. J. Appl. Oral. Sci. 2018, 26, e20170084. [Google Scholar] [CrossRef]
- Lee, D.; Wufuer, M.; Kim, I.; Choi, T.H.; Kim, B.J.; Jung, H.G.; Jeon, B.; Lee, G.; Jeon, O.H.; Chang, H.; et al. Sequential dual-drug delivery of BMP-2 and alendronate from hydroxyapatite-collagen scaffolds for enhanced bone regeneration. Sci. Rep. 2021, 11, 746. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Zhi, W.; Wang, J.; Feng, B.; Qu, S.; Mu, Y.; Weng, J. Immobilization of salvianolic acid B-loaded chitosan microspheres distributed three-dimensionally and homogeneously on the porous surface of hydroxyapatite scaffolds. Biomed. Mater. 2016, 11, 055014. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Zhang, R.; Huang, Y.; Li, Y.; Shan, M.; Zhong, X.; Xing, Y.; Wang, M.; Zhang, Y.; et al. Cartilage Extracellular Matrix Scaffold with Kartogenin-Encapsulated PLGA Microspheres for Cartilage Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 600103. [Google Scholar] [CrossRef]
- Zhu, K.; Zhao, F.; Yang, Y.; Mu, W. Effects of simvastatin-loaded PLGA microspheres on treatment of rats with intervertebral disk degeneration and on 6-K-PGF1alpha and HIF-1alpha. Exp. Ther. Med. 2020, 19, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, H.W.; Lee, J.H.; Oh, S.H. Controlling oxygen release from hollow microparticles for prolonged cell survival under hypoxic environment. Biomaterials 2015, 53, 583–591. [Google Scholar] [CrossRef]
- Guo, Z.; Bo, D.; He, P.; Li, H.; Wu, G.; Li, Z.; Zhou, C.; Li, Q. Sequential controlled-released dual-drug loaded scaffold for guided bone regeneration in a rat fenestration defect model. J. Mater. Chem. B 2017, 5, 7701–7710. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shen, G.; Shang, Q.; Zhang, Z.; Zhao, W.; Zhang, P.; Liang, D.; Ren, H.; Jiang, X. A Naringin-loaded gelatin-microsphere/nano-hydroxyapatite/silk fibroin composite scaffold promoted healing of critical-size vertebral defects in ovariectomised rat. Int. J. Biol. Macromol. 2021, 193 Pt A, 510–518. [Google Scholar] [CrossRef]
- Sun, Y.; Long, D. Preparation, Characterization and in vitro/in vivo Evaluation of Lovastatin-Loaded PLGA Microspheres by Local Administration for Femoral Head Necrosis. Drug Des. Devel. Ther. 2021, 15, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yun, H.S. Hydroxyapatite-containing gelatin/chitosan microspheres for controlled release of lysozyme and enhanced cytocompatibility. J. Mater. Chem. B 2014, 2, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, X.; Sun, X.; Long, C.; Sun, F.; Liu, J.; Li, X.; Lee, R.J.; Liu, N.; Li, Y.; et al. Ketoprofen and MicroRNA-124 Co-loaded poly (lactic-co-glycolic acid) microspheres inhibit progression of Adjuvant-induced arthritis in rats. Int. J. Pharm. 2018, 552, 148–153. [Google Scholar] [CrossRef]
- Wang, H.J.; Lin, Z.X.; Liu, X.M.; Sheng, S.Y.; Wang, J.Y. Heparin-loaded zein microsphere film and hemocompatibility. J. Control. Release 2005, 105, 120–131. [Google Scholar] [CrossRef]
- Xia, Q.; Yun, Y.; Li, Q.; Huang, Z.; Liang, Z. Preparation and characterization of monodisperse molecularly imprinted polymer microspheres by precipitation polymerization for kaempferol. Des. Monomers Polym. 2017, 20, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Wernecke, C.; Braun, H.J.; Dragoo, J.L. The Effect of Intra-articular Corticosteroids on Articular Cartilage: A Systematic Review. Orthop. J. Sports Med. 2015, 3, 2325967115581163. [Google Scholar] [CrossRef]
- Chauhan, N.; Gupta, P.; Arora, L.; Pal, D.; Singh, Y. Dexamethasone-loaded, injectable pullulan-poly(ethylene glycol) hydrogels for bone tissue regeneration in chronic inflammatory conditions. Mater. Sci. Eng. C 2021, 130, 112463. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Wang, L.; Sun, L.; Kim, B.Y.S.; Jiang, W.; Yuan, Y.; Liu, C. Spatiotemporal Immunomodulation Using Biomimetic Scaffold Promotes Endochondral Ossification-Mediated Bone Healing. Adv. Sci. 2021, 8, e2100143. [Google Scholar] [CrossRef]
- Paik, J.; Duggan, S.T.; Keam, S.J. Triamcinolone Acetonide Extended-Release: A Review in Osteoarthritis Pain of the Knee. Drugs 2019, 79, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Gan, L.; Zhang, T.; Ren, Q.; Sun, C. Melatonin alleviates adipose inflammation through elevating alpha-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J. Pineal Res. 2018, 64, e12455. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Wang, B.; Wen, H.; Smith, W.; Hao, D.; He, B.; Kong, L. Regulation effects of melatonin on bone marrow mesenchymal stem cell differentiation. J. Cell. Physiol. 2019, 234, 1008–1015. [Google Scholar] [CrossRef]
- Jarrar, H.; Altındal, D.Ç.; Gümüşderelioğlu, M. Effect of melatonin/BMP-2 co-delivery scaffolds on the osteoclast activity. J. Mater. Sci. Mater. Med. 2021, 32, 32. [Google Scholar] [CrossRef]
- Radio, N.M.; Doctor, J.S.; Witt-Enderby, P.A. Melatonin enhances alkaline phosphatase activity in differentiating human adult mesenchymal stem cells grown in osteogenic medium via MT2 melatonin receptors and the MEK/ERK (1/2) signaling cascade. J. Pineal Res. 2006, 40, 332–342. [Google Scholar] [CrossRef]
- Jarrar, H.; Altındal, D.Ç.; Gümüşderelioğlu, M. Scaffold-based osteogenic dual delivery system with melatonin and BMP-2 releasing PLGA microparticles. Int. J. Pharm. 2021, 600, 120489. [Google Scholar] [CrossRef]
- Huang, R.Y.; Hsiao, P.Y.; Mau, L.P.; Tsai, Y.C.; Cochran, D.L.; Weng, P.W.; Cheng, W.C.; Chung, C.H.; Huang, Y.C. Synthesis and Characterization of Melatonin-Loaded Chitosan Microparticles Promote Differentiation and Mineralization in Preosteoblastic Cells. J. Oral Implantol. 2020, 46, 562–570. [Google Scholar] [CrossRef]
- Safari, B.; Davaran, S.; Aghanejad, A. Osteogenic potential of the growth factors and bioactive molecules in bone regeneration. Int. J. Biol. Macromol. 2021, 175, 544–557. [Google Scholar] [CrossRef]
- Chen, X.; Tan, B.; Bao, Z.; Wang, S.; Tang, R.; Wang, Z.; Chen, G.; Chen, S.; Lu, W.W.; Yang, D.; et al. Enhanced bone regeneration via spatiotemporal and controlled delivery of a genetically engineered BMP-2 in a composite Hydrogel. Biomaterials 2021, 277, 121117. [Google Scholar] [CrossRef] [PubMed]
- Kang, F.; Yi, Q.; Gu, P.; Dong, Y.; Zhang, Z.; Zhang, L.; Bai, Y. Controlled growth factor delivery system with osteogenic-angiogenic coupling effect for bone regeneration. J. Orthop. Translat. 2021, 31, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Link, D.P.; van den Dolder, J.; van den Beucken, J.J.; Wolke, J.G.; Mikos, A.G.; Jansen, J.A. Bone response and mechanical strength of rabbit femoral defects filled with injectable CaP cements containing TGF-beta 1 loaded gelatin microparticles. Biomaterials 2008, 29, 675–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Yaszemski, M.J.; Mikos, A.G. TGF-beta1 release from biodegradable polymer microparticles: Its effects on marrow stromal osteoblast function. J. Bone Jt. Surg. Am. 2001, 83 (Suppl. 1), S82–S91. [Google Scholar] [CrossRef]
- Mackie, E.J.; Ahmed, Y.A.; Tatarczuch, L.; Chen, K.S.; Mirams, M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [Google Scholar] [CrossRef]
- Solorio, L.D.; Vieregge, E.L.; Dhami, C.D.; Dang, P.N.; Alsberg, E. Engineered cartilage via self-assembled hMSC sheets with incorporated biodegradable gelatin microspheres releasing transforming growth factor-β1. J. Control. Release 2012, 158, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Solorio, L.D.; Phillips, L.M.; McMillan, A.; Cheng, C.W.; Dang, P.N.; Samorezov, J.E.; Yu, X.; Murphy, W.L.; Alsberg, E. Spatially organized differentiation of mesenchymal stem cells within biphasic microparticle-incorporated high cell density osteochondral tissues. Adv. Healthc. Mater. 2015, 4, 2306–2313. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Tang, Y.; Zhao, K.; Zha, X.; Wei, M.; Tan, Q.; Wu, Z. Sequential release of double drug (graded distribution) loaded gelatin microspheres/PMMA bone cement. J. Mater. Chem. B 2021, 9, 508–522. [Google Scholar] [CrossRef]
- Min, J.; Choi, K.Y.; Dreaden, E.C.; Padera, R.F.; Braatz, R.D.; Spector, M.; Hammond, P.T. Designer Dual Therapy Nanolayered Implant Coatings Eradicate Biofilms and Accelerate Bone Tissue Repair. ACS Nano 2016, 10, 4441–4450. [Google Scholar] [CrossRef]
- Min, J.; Braatz, R.D.; Hammond, P.T. Tunable staged release of therapeutics from layer-by-layer coatings with clay interlayer barrier. Biomaterials 2014, 35, 2507–2517. [Google Scholar] [CrossRef]
- Asik, M.D.; Kaplan, M.; Yalinay, M.; Guven, E.O.; Bozkurt, M. Development of a Sequential Antibiotic Releasing System for Two-Stage Total Joint Replacement Surgery. J. Biomed. Nanotechnol. 2019, 15, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, H.; Vedantham, K.; Aniket; Young, A.; Marriott, I.; El-Ghannam, A. Tissue engineering scaffold for sequential release of vancomycin and rhBMP2 to treat bone infections. J. Biomed. Mater. Res. A 2014, 102, 4213–4223. [Google Scholar] [CrossRef]
- Sreeja, S.; Muraleedharan, C.V.; Varma, P.R.H.; Sailaja, G.S. Surface-transformed osteoinductive polyethylene terephthalate scaffold as a dual system for bone tissue regeneration with localized antibiotic delivery. Mater. Sci. Eng. C 2020, 109, 110491. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, R.; Izquierdo-Barba, I.; Vallet-Regi, M. 3D scaffold with effective multidrug sequential release against bacteria biofilm. Acta Biomater. 2017, 49, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Lee, C.C.; Wang, Y.P.; Chen, H.J.; Lai, C.H.; Hsieh, W.L.; Chen, Y.W. Controlled-release of tetracycline and lovastatin by poly(D,L-lactide-co-glycolide acid)-chitosan nanoparticles enhances periodontal regeneration in dogs. Int. J. Nanomed. 2016, 11, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Orellana, B.R.; Puleo, D.A. Tailored sequential drug release from bilayered calcium sulfate composites. Mater. Sci. Eng. C 2014, 43, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Sundararaj, S.C.; Thomas, M.V.; Peyyala, R.; Dziubla, T.D.; Puleo, D.A. Design of a multiple drug delivery system directed at periodontitis. Biomaterials 2013, 34, 8835–8842. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Kretlow, J.D.; Spicer, P.P.; Tabata, Y.; Demian, N.; Wong, M.E.; Kasper, F.K.; Mikos, A.G. Antibiotic-releasing porous polymethylmethacrylate/gelatin/antibiotic constructs for craniofacial tissue engineering. J. Control. Release 2011, 152, 196–205. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Jin, Y.; Wang, X.; Yao, S.; Li, Y.; Wu, Q.; Ma, G.; Cui, F.; Liu, H. An Antimicrobial Peptide-Loaded Gelatin/Chitosan Nanofibrous Membrane Fabricated by Sequential Layer-by-Layer Electrospinning and Electrospraying Techniques. Nanomaterials 2018, 8, 327. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wu, L.; Li, H.; Long, Z.; Song, X. Drug Release Characteristics and Tissue Distribution of Rifapentine Polylactic Acid Sustained-Release Microspheres in Rabbits after Paravertebral Implantation. Iran. Red Crescent Med. J. 2016, 18, e38661. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.R.; Jayakrishnan, A.; Kumar, T.S.S. Combinational delivery of anticancer drugs for osteosarcoma treatment using electrosprayed core shell nanocarriers. J. Mater. Sci. Mater. Med. 2020, 31, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, J.; Wang, C.; Zhang, Y.; Hu, C.; Li, G.; Xu, L. Application of HIF-1α by gene therapy enhances angiogenesis and osteogenesis in alveolar bone defect regeneration. J. Gene Med. 2016, 18, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, Z.; Dang, M.; Zhang, X.; Doleyres, Y.; Song, Y.; Chen, D.; Ma, P.X. Injectable nanofibrous spongy microspheres for NR4A1 plasmid DNA transfection to reverse fibrotic degeneration and support disc regeneration. Biomaterials 2017, 131, 86–97. [Google Scholar] [CrossRef] [Green Version]
- McMillan, A.; Nguyen, M.K.; Gonzalez-Fernandez, T.; Ge, P.; Yu, X.; Murphy, W.L.; Kelly, D.J.; Alsberg, E. Dual non-viral gene delivery from microparticles within 3D high-density stem cell constructs for enhanced bone tissue engineering. Biomaterials 2018, 161, 240–255. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, Y.; Zhu, W.; Zhang, G.; Yang, Y.P.; Zhao, C. The role of MicroRNAs in tendon injury, repair, and related tissue engineering. Biomaterials 2021, 277, 121083. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Leng, Q.; Chen, L.; Lv, Y. RNA-based scaffolds for bone regeneration: Application and mechanisms of mRNA, miRNA and siRNA. Theranostics 2020, 10, 3190–3205. [Google Scholar] [CrossRef]
- Wu, D.; Chang, X.; Tian, J.; Kang, L.; Wu, Y.; Liu, J.; Wu, X.; Huang, Y.; Gao, B.; Wang, H.; et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: Release of exosomal miR-1260a improves osteogenesis and angiogenesis. J. Nanobiotechnol. 2021, 19, 209. [Google Scholar] [CrossRef]
- Pankajakshan, D.; Agrawal, D. Mesenchymal Stem Cell Paracrine Factors in Vascular Repair and Regeneration. J. Biomed. Technol. Res. 2014, 1. [Google Scholar] [CrossRef] [Green Version]
- Sze, S.K.; de Kleijn, D.P.; Lai, R.C.; Tan, E.K.W.; Zhao, H.; Yeo, K.S.; Low, T.Y.; Lian, Q.; Lee, C.N.; Mitchell, W.; et al. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Mol. Cell. Proteom. 2007, 6, 1680–1689. [Google Scholar] [CrossRef] [Green Version]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Zamani, F.; Hajibaba, M.; Rasouli-Saravani, A.; Noroozbeygi, M.; Gorgani, M.; Hosseini-Fard, S.R.; Jalalifar, S.; Ajdarkosh, H.; Abedi, S.H.; et al. The pathogenic, therapeutic and diagnostic role of exosomal microrna in the autoimmune diseases. J. Neuroimmunol. 2021, 358, 577640. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Binabaj, M.M.; Ferns, G.A. Exosomes: Emerging modulators of signal transduction in colorectal cancer from molecular understanding to clinical application. Biomed. Pharmacother. 2021, 141, 111882. [Google Scholar] [CrossRef] [PubMed]
- Swanson, W.B.; Zhang, Z.; Xiu, K.; Gong, T.; Eberle, M.; Wang, Z.; Ma, P.X. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater. 2020, 118, 215–232. [Google Scholar] [CrossRef]
- Boraei, S.B.A.; Nourmohammadi, J.; Mahdavi, F.S.; Yus, J.; Ferrandez-Montero, A.; Sanchez-Herencia, A.J.; Gonzalez, Z.; Ferrari, B. Effect of SrR delivery in the biomarkers of bone regeneration during the in vitro degradation of HNT/GN coatings prepared by EPD. Colloids Surf. B Biointerfaces 2020, 190, 110944. [Google Scholar] [CrossRef]
- Gunputh, U.F.; Le, H.; Handy, R.D.; Tredwin, C. Anodised TiO2 nanotubes as a scaffold for antibacterial silver nanoparticles on titanium implants. Mater. Sci. Eng. C 2018, 91, 638–644. [Google Scholar] [CrossRef]
- Perez, R.A.; Kim, J.H.; Buitrago, J.O.; Wall, I.B.; Kim, H.W. Novel therapeutic core-shell hydrogel scaffolds with sequential delivery of cobalt and bone morphogenetic protein-2 for synergistic bone regeneration. Acta Biomater. 2015, 23, 295–308. [Google Scholar] [CrossRef]
- Li, D.; Chen, K.; Duan, L.; Fu, T.; Li, J.; Mu, Z.; Wang, S.; Zou, Q.; Chen, L.; Feng, Y.; et al. Strontium Ranelate Incorporated Enzyme-Cross-Linked Gelatin Nanoparticle/Silk Fibroin Aerogel for Osteogenesis in OVX-Induced Osteoporosis. ACS Biomater. Sci. Eng. 2019, 5, 1440–1451. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, Q.; Li, Y.; Fan, J.; Wang, G.; Pan, H.; Ruan, C. Strontium incorporation improves the bone-forming ability of scaffolds derived from porcine bone. Colloids Surf. B Biointerfaces 2018, 162, 279–287. [Google Scholar] [CrossRef]
- Kwon, Y.D.; Yang, D.H.; Lee, D.W. A Titanium Surface-Modified with Nano-Sized Hydroxyapatite and Simvastatin Enhances Bone Formation and Osseintegration. J. Biomed. Nanotechnol. 2015, 11, 1007–1015. [Google Scholar] [CrossRef]
- Rather, H.A.; Patel, R.; Yadav, U.C.; Vasita, R. Dual drug-delivering polycaprolactone-collagen scaffold to induce early osteogenic differentiation and coupled angiogenesis. Biomed. Mater. 2020, 15, 045008. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.C.; Dovban, A.S.; Lim, L.P.; Chong, L.Y.; Kuo, M.Y.; Wang, C.H. Dual delivery of PDGF and simvastatin to accelerate periodontal regeneration in vivo. Biomaterials 2013, 34, 9990–9997. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.C.; Chong, L.Y.; Dovban, A.S.; Lim, L.P.; Lim, J.C.; Kuo, M.Y.; Wang, C.H. Sequential platelet-derived growth factor-simvastatin release promotes dentoalveolar regeneration. Tissue Eng. Part A 2014, 20, 356–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.C.; Lim, L.P.; Chong, L.Y.; Dovban, A.S.; Chien, L.Y.; Chung, M.C.; Lei, C.; Kao, M.J.; Chen, C.H.; Chiang, H.C.; et al. PDGF-simvastatin delivery stimulates osteogenesis in heat-induced osteonecrosis. J. Dent. Res. 2012, 91, 618–624. [Google Scholar] [CrossRef]
- Shu, X.; Feng, J.; Feng, J.; Huang, X.; Li, L.; Shi, Q. Combined delivery of bone morphogenetic protein-2 and insulin-like growth factor-1 from nano-poly (gamma-glutamic acid)/beta-tricalcium phosphate-based calcium phosphate cement and its effect on bone regeneration in vitro. J. Biomater. Appl. 2017, 32, 547–560. [Google Scholar] [CrossRef]
- Zhang, W.; Ling, C.; Zhang, A.; Liu, H.; Jiang, Y.; Li, X.; Sheng, R.; Yao, Q.; Chen, J. An all-silk-derived functional nanosphere matrix for sequential biomolecule delivery and in situ osteochondral regeneration. Bioact. Mater. 2020, 5, 832–843. [Google Scholar] [CrossRef]
- Hwang, S.C.; Hwang, D.S.; Kim, H.Y.; Kim, M.J.; Kang, Y.H.; Byun, S.H.; Rho, G.J.; Lee, H.J.; Lee, H.C.; Kim, S.H.; et al. Development of bone regeneration strategies using human periosteum-derived osteoblasts and oxygen-releasing microparticles in mandibular osteomyelitis model of miniature pig. J. Biomed. Mater. Res. A 2019, 107, 2183–2194. [Google Scholar] [CrossRef]
- Yu, P.; Yu, F.; Xiang, J.; Zhou, K.; Zhou, L.; Zhang, Z.; Rong, X.; Ding, Z.; Wu, J.; Li, W.; et al. Mechanistically Scoping Cell-free and Cell-dependent Artificial Scaffolds in Rebuilding Skeletal and Dental Hard Tissues. Adv. Mater. 2021, 34, 2107922. [Google Scholar] [CrossRef]
- Cao, H.; Wang, X.; Chen, M.; Liu, Y.; Cui, X.; Liang, J.; Wang, Q.; Fan, Y.; Zhang, X. Childhood Cartilage ECM Enhances the Chondrogenesis of Endogenous Cells and Subchondral Bone Repair of the Unidirectional Collagen-dECM Scaffolds in Combination with Microfracture. ACS Appl. Mater. Interfaces 2021, 13, 57043–57057. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, J.Y.; Tong, X.M.; Mei, J.G.; Chen, Y.F.; Mou, X.Z. Recent advances in the use of microcarriers for cell cultures and their ex vivo and in vivo applications. Biotechnol. Lett. 2020, 42, 1–10. [Google Scholar] [CrossRef]
- Indana, D.; Agarwal, P.; Bhutani, N.; Chaudhuri, O. Viscoelasticity and Adhesion Signaling in Biomaterials Control Human Pluripotent Stem Cell Morphogenesis in 3D Culture. Adv. Mater. 2021, 33, e2101966. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.-N.; Vu, N.B.; Nguyen, P.D.-N.; Dao, T.T.-T.; To, X.H.-V.; Van Pham, P. In vitro cartilage differentiation of human adipose-derived mesenchymal stem cell spheroids cultured in porous scaffolds. Front. Biosci. 2021, 26, 266–285. [Google Scholar] [CrossRef]
- Shanbhag, S.; Suliman, S.; Mohamed-Ahmed, S.; Kampleitner, C.; Hassan, M.N.; Heimel, P.; Dobsak, T.; Tangl, S.; Bolstad, A.I.; Mustafa, K. Bone regeneration in rat calvarial defects using dissociated or spheroid mesenchymal stromal cells in scaffold-hydrogel constructs. Stem Cell Res. Ther. 2021, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, M.; Meng, F.; Liu, Z.; Wang, S.; Zhang, Y.; Li, M.; Li, Z.; Zhang, L.; Tang, P. 3D spheroids of human placenta-derived mesenchymal stem cells attenuate spinal cord injury in mice. Cell Death Dis. 2021, 12, 1096. [Google Scholar] [CrossRef]
- Ylöstalo, J.H.; Bartosh, T.J.; Coble, K.; Prockop, D.J. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells 2012, 30, 2283–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, K.; Zhu, Y.; Shi, Y.; Chen, T.; Wang, H.; Guo, Y.; Deng, P.; Liu, H.; Shao, X.; Qin, J. Establishment of Trophoblast-Like Tissue Model from Human Pluripotent Stem Cells in Three-Dimensional Culture System. Adv. Sci. 2022, 9, e2100031. [Google Scholar] [CrossRef]
- Passanha, F.R.; Gomes, D.B.; Piotrowska, J.; Moroni, L.; Baker, M.B.; LaPointe, V.L.S. A comparative study of mesenchymal stem cells cultured as cell-only aggregates and in encapsulated hydrogels. J. Tissue Eng. Regen. Med. 2022, 16, 14–25. [Google Scholar] [CrossRef]
- Barooji, Y.F.; Hvid, K.G.; Petitjean, I.I.; Brickman, J.M.; Oddershede, L.B.; Bendix, P.M. Changes in Cell Morphology and Actin Organization in Embryonic Stem Cells Cultured under Different Conditions. Cells 2021, 10, 2859. [Google Scholar] [CrossRef]
- Schmitz, C.; Potekhina, E.; Belousov, V.V.; Lavrentieva, A. Hypoxia Onset in Mesenchymal Stem Cell Spheroids: Monitoring with Hypoxia Reporter Cells. Front. Bioeng. Biotechnol. 2021, 9, 611837. [Google Scholar] [CrossRef]
- Chen, L.C.; Wang, H.W.; Huang, C.C. Modulation of Inherent Niches in 3D Multicellular MSC Spheroids Reconfigures Metabolism and Enhances Therapeutic Potential. Cells 2021, 10, 2747. [Google Scholar] [CrossRef]
- Hu, W.; Lazar, M. Modelling metabolic diseases and drug response using stem cells and organoids. Nat. Rev. Endocrinol. 2022, 18, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Cheong, E.; Kwak, J.; Carpenter, R.; Shim, J.; Lee, J. Trabecular bone organoid model for studying the regulation of localized bone remodeling. Sci. Adv. 2021, 7, eabd6495. [Google Scholar] [CrossRef] [PubMed]
- Mielan, B.; Sousa, D.M.; Krok-Borkowicz, M.; Eloy, P.; Dupont, C.; Lamghari, M.; Pamuła, E. Polymeric Microspheres/Cells/Extracellular Matrix Constructs Produced by Auto-Assembly for Bone Modular Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 7897. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Gao, Q.; Zhao, H.; Nie, J.; Fu, Z.; Wang, H.; Chen, L.; Shao, L.; Fu, J.; Chen, Z.; et al. Electro-Assisted Bioprinting of Low-Concentration GelMA Microdroplets. Small 2019, 15, e1804216. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.S.; Jain, E.; Kolar, G.; Kim, Y.; Sell, S.A.; Zustiak, S.P. Design of electrohydrodynamic sprayed polyethylene glycol hydrogel microspheres for cell encapsulation. Biofabrication 2017, 9, 025019. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Ghanian, M.H.; Baharvand, H.; Vahidi, B.; Eslaminejad, M.B. Engineering mesenchymal stem cell spheroids by incorporation of mechanoregulator microparticles. J. Mech. Behav. Biomed. Mater. 2018, 84, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Sart, S.; Tsai, A.C.; Li, Y.; Ma, T. Three-dimensional aggregates of mesenchymal stem cells: Cellular mechanisms, biological properties, and applications. Tissue Eng. Part B Rev. 2014, 20, 365–380. [Google Scholar] [CrossRef] [Green Version]
- Turlier, H.; Maître, J.L. Mechanics of tissue compaction. Semin. Cell Dev. Biol. 2015, 47–48, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, S.; Sasaki, Y.; Kubo, H.; Sawada, S.I.; Kinoshita, N.; Marukawa, E.; Harada, H.; Akiyoshi, K. Construction of Hybrid Cell Spheroids Using Cell-Sized Cross-Linked Nanogel Microspheres as an Artificial Extracellular Matrix. ACS Appl. Bio. Mater. 2021, 4, 7848–7855. [Google Scholar] [CrossRef]
- Daly, A.C.; Davidson, M.D.; Burdick, J.A. 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nat. Commun. 2021, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhang, J.; Liu, J.; Yu, Z.Y. Granular hydrogels for 3D bioprinting applications. View 2020, 1, 20200060. [Google Scholar] [CrossRef]
- Riley, L.; Schirmer, L.; Segura, T. Granular hydrogels: Emergent properties of jammed hydrogel microparticles and their applications in tissue repair and regeneration. Curr. Opin. Biotechnol. 2019, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ataie, Z.; Kheirabadi, S.; Zhang, J.W.; Kedzierski, A.; Petrosky, C.; Jiang, R.; Vollberg, C.; Sheikhi, A. Nanoengineered Granular Hydrogel Bioinks with Preserved Interconnected Microporosity for Extrusion Bioprinting. Small 2022, 18, e2202390. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Jeon, O.; Cleveland, D.; Gasvoda, K.L.; Wells, D.; Lee, S.J.; Alsberg, E. Jammed Micro-Flake Hydrogel for Four-Dimensional Living Cell Bioprinting. Adv. Mater. 2022, 34, e2109394. [Google Scholar] [CrossRef] [PubMed]
- Mikael, P.E.; Golebiowska, A.A.; Xin, X.; Rowe, D.W.; Nukavarapu, S.P. Evaluation of an Engineered Hybrid Matrix for Bone Regeneration via Endochondral Ossification. Ann. Biomed. Eng. 2020, 48, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.N.; Dwivedi, N.; Phillips, L.M.; Yu, X.; Herberg, S.; Bowerman, C.; Solorio, L.D.; Murphy, W.L.; Alsberg, E. Controlled Dual Growth Factor Delivery From Microparticles Incorporated within Human Bone Marrow-Derived Mesenchymal Stem Cell Aggregates for Enhanced Bone Tissue Engineering via Endochondral Ossification. Stem Cells Transl. Med. 2016, 5, 206–217. [Google Scholar] [CrossRef]
- Xie, C.; Liang, R.; Ye, J.; Peng, Z.; Sun, H.; Zhu, Q.; Shen, X.; Hong, Y.; Wu, H.; Sun, W.; et al. High-efficient engineering of osteo-callus organoids for rapid bone regeneration within one month. Biomaterials 2022, 288, 121741. [Google Scholar] [CrossRef]
- Ji, X.; Shao, H.; Li, X.; Ullah, M.W.; Luo, G.; Xu, Z.; Ma, L.; He, X.; Lei, Z.; Li, Q.; et al. Injectable immunomodulation-based porous chitosan microspheres/HPCH hydrogel composites as a controlled drug delivery system for osteochondral regeneration. Biomaterials 2022, 285, 121530. [Google Scholar] [CrossRef]
- Neto, M.D.; Oliveira, M.B.; Mano, J.F. Microparticles in Contact with Cells: From Carriers to Multifunctional Tissue Modulators. Trends Biotechnol. 2019, 37, 1011–1028. [Google Scholar] [CrossRef]
- Lin, Z.; Shen, D.; Zhou, W.; Zheng, Y.; Kong, T.; Liu, X.; Wu, S.; Chu, P.K.; Zhao, Y.; Wu, J.; et al. Regulation of extracellular bioactive cations in bone tissue microenvironment induces favorable osteoimmune conditions to accelerate in situ bone regeneration. Bioact. Mater. 2021, 6, 2315–2330. [Google Scholar] [CrossRef]
- Tan, S.; Wang, Y.; Du, Y.; Xiao, Y.; Zhang, S. Injectable bone cement with magnesium-containing microspheres enhances osteogenesis via anti-inflammatory immunoregulation. Bioact. Mater. 2021, 6, 3411–3423. [Google Scholar] [CrossRef]
- Wu, L.; Xu, Y.; Xi, K.; Gu, Y.; Tang, J.; Xin, T.; Yang, H.; Wang, L.; Cui, W.; Chen, L. Regulation of macrophage subtype via injectable micro/nano-structured porous microsphere for reprogramming osteoimmune microenvironment. Chem. Eng. J. 2022, 439, 135692. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Z.; Chen, Y.; Guan, M.X. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell 2017, 8, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Liang, J.; Zhu, Y.; Hu, M.; Deng, L.; Cui, W.; Xu, X. Fullerol-hydrogel microfluidic spheres for in situ redox regulation of stem cell fate and refractory bone healing. Bioact. Mater. 2021, 6, 4801–4815. [Google Scholar] [CrossRef]
- Zheng, L.; Zhuang, Z.; Li, Y.; Shi, T.; Fu, K.; Yan, W.; Zhang, L.; Wang, P.; Li, L.; Jiang, Q. Bone targeting antioxidative nano-iron oxide for treating postmenopausal osteoporosis. Bioact. Mater. 2022, 14, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Kim, J. ROS-Scavenging Therapeutic Hydrogels for Modulation of the Inflammatory Response. ACS Appl. Mater. Interfaces 2021, 14, 23002–23021. [Google Scholar] [CrossRef] [PubMed]
- Chotchindakun, K.; Pekkoh, J.; Ruangsuriya, J.; Zheng, K.; Unalan, I.; Boccaccini, A.R. Fabrication and Characterization of Cinnamaldehyde-Loaded Mesoporous Bioactive Glass Nanoparticles/PHBV-Based Microspheres for Preventing Bacterial Infection and Promoting Bone Tissue Regeneration. Polymers 2021, 13, 1794. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Yuan, Z.; Cai, Q.; Mao, J.; Yang, X. Bioresorbable Microspheres with Surface-Loaded Nanosilver and Apatite as Dual-Functional Injectable Cell Carriers for Bone Regeneration. Macromol. Rapid Commun. 2018, 39, e1800062. [Google Scholar] [CrossRef]
- Huang, Y.; Du, Z.; Zheng, T.; Jing, W.; Liu, H.; Liu, X.; Mao, J.; Zhang, X.; Cai, Q.; Chen, D.; et al. Antibacterial, conductive, and osteocompatible polyorganophosphazene microscaffolds for the repair of infectious calvarial defect. J. Biomed. Mater. Res. A 2021, 109, 2580–2596. [Google Scholar] [CrossRef]
- Meng, D.; Francis, L.; Thompson, I.D.; Mierke, C.; Huebner, H.; Amtmann, A.; Roy, I.; Boccaccini, A.R. Tetracycline-encapsulated P(3HB) microsphere-coated 45S5 Bioglass(®)-based scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 2809–2817. [Google Scholar] [CrossRef]
- Farzin, A.; Etesami, S.A.; Goodarzi, A.; Ai, J. A facile way for development of three-dimensional localized drug delivery system for bone tissue engineering. Mater. Sci. Eng. C 2019, 105, 110032. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lu, J.; Ke, Q.; Peng, X.; Guo, Y.; Xie, X. Magnetic Mesoporous Calcium Sillicate/Chitosan Porous Scaffolds for Enhanced Bone Regeneration and Photothermal-Chemotherapy of Osteosarcoma. Sci. Rep. 2018, 8, 7345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.Y.; Hu, Y.; Li, L.; Wang, C.; Wang, J.; Li, Y.; Chen, D.; Ding, X.; Shen, C.; Xu, F.J. Biomass-Derived Multilayer-Structured Microparticles for Accelerated Hemostasis and Bone Repair. Adv. Sci. 2020, 7, 2002243. [Google Scholar] [CrossRef] [PubMed]
- Yadavali, S.; Jeong, H.H.; Lee, D.; Issadore, D. Silicon and glass very large scale microfluidic droplet integration for terascale generation of polymer microparticles. Nat. Commun. 2018, 9, 1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, J.E.; Avila, C.F.; Hall, S.A.; Lenoir, N.; Viggiani, G. Multiscale modeling and characterization of granular matter: From grain kinematics to continuum mechanics. J. Mech. Phys. Solids 2011, 59, 237–250. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Z.; Guo, M.; Pan, C.; Huang, Z.; Jin, J.; Li, Y.; Hou, X.; Li, W. Biomimetic Hydrogels Loaded with Nanofibers Mediate Sustained Release of pDNA and Promote In Situ Bone Regeneration. Macromol. Biosci. 2021, 21, e2000393. [Google Scholar] [CrossRef]


| Type | Name | Reference |
|---|---|---|
| Growth factors | bone morphogenic protein-2 (BMP-2) | [83] |
| bone morphogenetic factor-6 (BMP-6) | [84] | |
| interleukin-4 (IL-4) | [85] | |
| vascular endothelial growth factor (VEGF) | [59] | |
| insulin-like growth factor (IGF-1) | [86] | |
| fibroblast growth factor (FGF) | [87] | |
| fibroblast growth factor-2 (FGF-2) | [88] | |
| osteogenic growth peptide (OGP) | [89] | |
| stromal cell derived factor-1 (SDF-1) | [90] | |
| platelet-derived growth factor (PDGF) | [91] | |
| transforming growth factor-β1 (TGF-β1) | [92] | |
| Antibiotics | gentamicin sulfate (GS) | [93] |
| ciprofloxacin | [94] | |
| Vancomycin | [95] | |
| levofloxacin | [96] | |
| gentamicin | [97] | |
| tetracycline | [98] | |
| metronidazole | [99] | |
| doxycycline | [100] | |
| colistin | [101] | |
| Antineoplastic drugs | methotrexate (MTX) | [102] |
| doxorubicin (DOX) | [103] | |
| paclitaxel (PTX) | [104] | |
| etoposide (ETP) | [105] | |
| Anti-tuberculosis drugs | isoniazid | [106] |
| rifampin | [107] | |
| Hormone | dexamethasone | [108] |
| melatonin | [109] | |
| Biologics | non-coding microRNA | [110] |
| exosomes | [111] | |
| Metals | trontium ranelate (SrR) | [112] |
| silver nanoparticles (Ag NPs) | [113] | |
| Co ions | [114] | |
| Strontium (Sr) | [115] | |
| Others | alendronate (ALN) | [116] |
| salvianolic acid B (SB) | [117] | |
| Kartogenin (KGN) | [118] | |
| simvastatin | [119] | |
| oxygen carrier perfluorooctane (PFO) | [120] | |
| parthenolide | [121] | |
| naringin | [122] | |
| lovastatin | [123] | |
| nano-sized hydroxyapatite (HAP) | [124] | |
| ketoprofen | [125] | |
| heparin | [126] | |
| kaempferol (KEM) | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Z.; Su, X.; Wang, T.; Sun, X.; Yang, H.; Guo, S. The Role of Microsphere Structures in Bottom-Up Bone Tissue Engineering. Pharmaceutics 2023, 15, 321. https://doi.org/10.3390/pharmaceutics15020321
Feng Z, Su X, Wang T, Sun X, Yang H, Guo S. The Role of Microsphere Structures in Bottom-Up Bone Tissue Engineering. Pharmaceutics. 2023; 15(2):321. https://doi.org/10.3390/pharmaceutics15020321
Chicago/Turabian StyleFeng, Ziyi, Xin Su, Ting Wang, Xiaoting Sun, Huazhe Yang, and Shu Guo. 2023. "The Role of Microsphere Structures in Bottom-Up Bone Tissue Engineering" Pharmaceutics 15, no. 2: 321. https://doi.org/10.3390/pharmaceutics15020321
APA StyleFeng, Z., Su, X., Wang, T., Sun, X., Yang, H., & Guo, S. (2023). The Role of Microsphere Structures in Bottom-Up Bone Tissue Engineering. Pharmaceutics, 15(2), 321. https://doi.org/10.3390/pharmaceutics15020321