A Supine Position and Dual-Dose Applications Enhance Spray Dosing to the Posterior Nose: Paving the Way for Mucosal Immunization
Abstract
:1. Introduction
- (1)
- Visualize the spatiotemporal development of the nasal sprays from different devices using a high-speed camera.
- (2)
- Visualize the liquid film translocation in the nose with unidirectional delivery for various administration angles, head positions, and inhalation flow rates.
- (3)
- Visualize the liquid film translocation with bidirectional delivery for various administration angles, head positions, inhalation flow rates, and the number of applications.
- (4)
- Quantify the nasal spray deposition in the front nose, turbinate, and nasopharynx.
- (5)
- Compare the performances among test cases and identify the optimal combination of the nasal device, delivery method, administration angle, inhalation flow rate, head position, and number of spray applications.
2. Materials and Methods
2.1. Study Design
2.2. Nasal Airway Casts and Computational Model
2.3. Visualization of Spray Plumes in Open Space
2.4. UV-Illuminated Fluorescent Visualization of Spray Dynamics within the Nose
2.5. Regional Dose Quantification
2.6. Statistical Analysis
3. Results
3.1. High-Speed Imaging of Soft-Mist and Squeeze-Bottle Sprays
3.2. Unidirectional Delivery
3.3. Bidirectional Delivery
3.3.1. Effects of Spray Administration Angles (Relative to the Nostril)
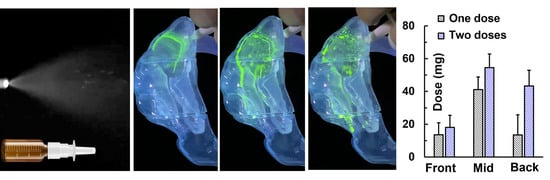
3.3.2. Effects of Dual-Spray Applications on Dosimetry
3.3.3. Head Positions (Supine)
3.4. Revisiting Unidirectional Delivery
Effects of Spray Administration Angles (Relative to the Nostril)
4. Discussion
4.1. Has the New Delivery System Delivered Sufficient Doses to the Posterior Nose?
4.2. Mechanisms Underlying Successful Posterior Nose Delivery
4.3. Bidirectional vs. Unidirectional Modes
4.4. Limitations
5. Conclusions
- (1)
- For nasal spray delivery, liquid film translocation can be a more important factor than the initial deposition in determining the dosimetry distribution.
- (2)
- Liquid film translocation is sensitive to the inhalation flow rate and head position.
- (3)
- Liquid film translocation is more sensitive to the inhalation flow rate in bidirectional delivery than in unidirectional delivery and in a supine position than in the upright position.
- (4)
- Factors favorable for posterior nose delivery include (1) flow shear mobilizing the liquid film, (2) gravity aligning with the target, and (3) slanted turbinate furrows assisting film motion and minimizing the wall liquid-holding capacity.
- (5)
- A supine position and dual-dose application significantly enhance nasal spray deposition in the caudal turbinate and nasopharynx. A nasopharynx deposition fraction of 31% was achieved vs. no nasopharynx deposition in an upright position with a one-dose application.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.T.; Nakayama, T.; Wu, C.-T.; Goltsev, Y.; Jiang, S.; Gall, P.A.; Liao, C.-K.; Shih, L.-C.; Schürch, C.M.; McIlwain, D.R.; et al. ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs. Nat. Commun. 2020, 11, 5453. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 receptor ACE2 Is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020, 181, 1016–1035.e1019. [Google Scholar] [CrossRef]
- Xu, K.; Shi, X.; Husted, C.; Hong, R.; Wang, Y.; Ning, B.; Sullivan, T.; Rieger-Christ, K.; Duan, F.; Marques, H.; et al. Smoking Modulates Different Secretory Subpopulations Expressing SARS-CoV-2 Entry Genes in the Nasal and Bronchial Airways. Res. Sq. 2021, 12, 18168. [Google Scholar] [CrossRef] [PubMed]
- Berger, G.; Marom, Z.; Ophir, D. Goblet cell density of the inferior turbinates in patients with perennial allergic and nonallergic rhinitis. Am. J. Rhinol. 1997, 11, 233–236. [Google Scholar] [CrossRef]
- Glezen, W.P. The new nasal spray influenza vaccine. Pediatr. Infect. Dis. J. 2001, 20, 731–732. [Google Scholar] [CrossRef]
- Xi, J. Development and challenges of nasal spray vaccines for short-term COVID-19 protection. Curr. Pharm. Biotechnol. 2022, 23, 1671–1677. [Google Scholar] [CrossRef]
- Nian, X.; Zhang, J.; Huang, S.; Duan, K.; Li, X.; Yang, X. Development of Nasal Vaccines and the Associated Challenges. Pharmaceutics 2022, 14, 1983. [Google Scholar] [CrossRef]
- Waltz, E. How nasal-spray vaccines could change the pandemic. Nature 2022, 609, 240–242. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Vora, L.K.; Singla, R.K.; Shah, P.; Uversky, V.N.; Apostolopoulos, V. Nitric oxide and its derivatives containing nasal spray and inhalation therapy for the treatment of COVID-19. Curr. Pharm. Des. 2022, 609, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Paull, J.R.A.; Luscombe, C.A.; Castellarnau, A.; Heery, G.P.; Bobardt, M.D.; Gallay, P.A. Protective Effects of Astodrimer Sodium 1% Nasal Spray Formulation against SARS-CoV-2 Nasal Challenge in K18-hACE2 Mice. Viruses 2021, 13, 1656. [Google Scholar] [CrossRef]
- Xi, J.; Yuan, J.E.; Zhang, Y.; Nevorski, D.; Wang, Z.; Zhou, Y. Visualization and quantification of nasal and olfactory deposition in a sectional adult nasal airway cast. Pharm. Res. 2016, 33, 1527–1541. [Google Scholar] [CrossRef]
- Xi, J.; Si, X.A.; Kim, J.; Zhang, Y.; Jacob, R.E.; Kabilan, S.; Corley, R.A. Anatomical details of the rabbit nasal passages and their implications in breathing, air conditioning, and olfaction. Anat. Rec. 2016, 299, 853–868. [Google Scholar] [CrossRef] [Green Version]
- Inthavong, K.; Fung, M.C.; Yang, W.; Tu, J. Measurements of droplet size distribution and analysis of nasal spray atomization from different actuation pressure. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.S.; Holmes, T.D.; Gao, J.; Guilmette, R.A.; Li, S.; Surakitbanharn, Y.; Rowlings, C. Characterization of nasal spray pumps and deposition pattern in a replica of the human nasal airway. J. Aerosol Med. 2001, 14, 267–280. [Google Scholar] [CrossRef]
- Kundoor, V.; Dalby, R.N. Effect of formulation- and administration-related variables on deposition pattern of nasal spray pumps evaluated using a nasal cast. Pharm. Res. 2011, 28, 1895–1904. [Google Scholar] [CrossRef]
- Si, X.A.; Sami, M.; Xi, J. Liquid film translocation significantly enhances nasal spray delivery to olfactory region: A numerical simulation study. Pharmaceutics 2021, 13, 903. [Google Scholar] [CrossRef]
- Abdelalim, A.A.; Mohamady, A.A.; Elsayed, R.A.; Elawady, M.A.; Ghallab, A.F. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: A randomized controlled trial. Am. J. Otolaryngol. 2021, 42, 102884. [Google Scholar] [CrossRef] [PubMed]
- Guenezan, J.; Garcia, M.; Strasters, D.; Jousselin, C.; Lévêque, N.; Frasca, D.; Mimoz, O. Povidone Iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: A randomized clinical trial. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 400–401. [Google Scholar] [CrossRef]
- Rubin, R. COVID-19 Vaccine Nasal Spray. Jama 2021, 326, 1138. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Si, X.A. A next-generation vaccine for broader and long-lasting COVID-19 protection. MedComm (2020) 2022, 3, e138. [Google Scholar] [CrossRef] [PubMed]
- Hoseini-Tavassol, Z.; Ejtahed, H.S.; Soroush, A.R.; Sajjadpour, Z.; Hasani-Ranjbar, S.; Larijani, B. Natural derived nasal spray: A proposed approach for COVID-19 disease control. Infect. Disord. Drug Targets 2021, 21, e160921191568. [Google Scholar] [CrossRef] [PubMed]
- Ku, Z.; Xie, X.; Hinton, P.R.; Liu, X.; Ye, X.; Muruato, A.E.; Ng, D.C.; Biswas, S.; Zou, J.; Liu, Y.; et al. Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants. Nature 2021, 595, 718–723. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vora, L.K.; Pandya, A.K.; Patravale, V.B. Intranasal vaccines for SARS-CoV-2: From challenges to potential in COVID-19 management. Drug Discov. Today 2021, 26, 2619–2636. [Google Scholar] [CrossRef]
- Xi, J.; Wang, Z.; Nevorski, D.; White, T.; Zhou, Y. Nasal and olfactory deposition with normal and bidirectional intranasal delivery techniques: In vitro tests and numerical simulations. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 118–131. [Google Scholar] [CrossRef]
- Xi, J.; Kim, J.; Si, X.A.; Corley, R.A.; Zhou, Y. Modeling of inertial deposition in scaled models of rat and human nasal airways: Towards in vitro regional dosimetry in small animals. J. Aerosol Sci. 2016, 99, 78–93. [Google Scholar] [CrossRef]
- Ochowiak, M.; Włodarczak, S.; Krupińska, A.; Matuszak, M. Particle Image Velocimetry based on Matlab and PIVlab for testing flow disturbing elements. In Design, Simulation, Manufacturing: The Innovation Exchange; Springer: Cham, Switzerland, 2021; pp. 268–276. [Google Scholar]
- Xi, J.; Lei, L.R.; Zouzas, W.; April Si, X. Nasally inhaled therapeutics and vaccination for COVID-19: Developments and challenges. MedComm 2021, 2, 569–586. [Google Scholar] [CrossRef]
- Moakes, R.J.A.; Davies, S.P.; Stamataki, Z.; Grover, L.M. Formulation of a Composite Nasal Spray Enabling Enhanced Surface Coverage and Prophylaxis of SARS-CoV-2. Adv. Mater. 2021, 33, e2008304. [Google Scholar] [CrossRef]
- Lin, Y.; Yue, S.; Yang, Y.; Yang, S.; Pan, Z.; Yang, X.; Gao, L.; Zhou, J.; Li, Z.; Hu, L.; et al. Nasal spray of neutralizing monoclonal antibody 35B5 confers potential prophylaxis against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs): A small-scale clinical trial. Clin. Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Carrageenan nasal spray may double the rate of recovery from coronavirus and influenza virus infections: Re-analysis of randomized trial data. Pharmacol. Res. Perspect. 2021, 9, e00810. [Google Scholar] [CrossRef] [PubMed]
- Castellarnau, A.; Heery, G.P.; Seta, A.; Luscombe, C.A.; Kinghorn, G.R.; Button, P.; McCloud, P.; Paull, J.R.A. Astodrimer sodium antiviral nasal spray for reducing respiratory infections is safe and well tolerated in a randomized controlled trial. Sci. Rep. 2022, 12, 10210. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Goodey, A.P.; Fang, X.; Jacob, K. A Comparison of the Deposition Patterns of Different Nasal Spray Formulations Using a Nasal Cast. Aerosol Sci. Technol. 2014, 48, 930–938. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Shen, X.; Mao, S. Factors influencing drug deposition in thenasal cavity upon delivery via nasal sprays. J. Pharm. Investig. 2020, 50, 251–259. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Rapiejko, P.; Sova, J.; Dobrowolska, K. Impact of physicochemical properties of nasal spray products on drug deposition and transport in the pediatric nasal cavity model. Int. J. Pharm. 2020, 574, 118911. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.L.; Pandey, M.; Choudhury, H.; Lim, W.M.; Bhattamisra, S.K.; Gorain, B. Development of In-Situ Spray for Local Delivery of Antibacterial Drug for Hidradenitis Suppurativa: Investigation of Alternative Formulation. Polymers 2021, 13, 2770. [Google Scholar] [CrossRef]
- Chaturvedi, M.; Kumar, M.; Pathak, K. A review on mucoadhesive polymer used in nasal drug delivery system. J. Adv. Pharm. Technol. Res. 2011, 2, 215–222. [Google Scholar] [CrossRef]
- Rygg, A.; Hindle, M.; Longest, P.W. Absorption and Clearance of Pharmaceutical Aerosols in the Human Nose: Effects of Nasal Spray Suspension Particle Size and Properties. Pharm. Res. 2016, 33, 909–921. [Google Scholar] [CrossRef]
- Han, K.; Woghiren, O.E.; Priefer, R. Surface tension examination of various liquid oral, nasal, and ophthalmic dosage forms. Chem. Cent. J. 2016, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Katsiavria, A.; Bontozoglou, V. Stability of liquid film flow laden with the soluble surfactant sodium dodecyl sulphate: Predictions versus experimental data. J. Fluid Mech. 2020, 894, A18. [Google Scholar] [CrossRef]
- Mirjalili, S.; Chan, W.H.R. Linear stability of a thin fluid film interacting with its surrounding bulk fluid. Phys. Fluids 2021, 33, 072104. [Google Scholar] [CrossRef]
- Craster, R.V.; Matar, O.K. Dynamics and stability of thin liquid films. Rev. Mod. Phys. 2009, 81, 1131–1198. [Google Scholar] [CrossRef]
- Yu, Y.Q.; Cheng, X. Experimental study of water film flow on large vertical and inclined flat plate. Prog. Nucl. Energy 2014, 77, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-S.; Kim, M.-U. The flow and hydrodynamic stability of a liquid film on a rotating disc. Fluid Dyn. Res. 2009, 41, 035504. [Google Scholar] [CrossRef]
- Steinberg, C.; Liu, M.; Hung, D.L.S. A combined experimental–numerical study towards the elucidation of spray–wall interaction on step geometries. Eng. Appl. Comput. Fluid Mech. 2022, 16, 1866–1882. [Google Scholar] [CrossRef]
- Du, R.; Zhang, A.; Du, Z.; Zhang, X. Molecular dynamics simulation on thin-film lubrication of a mixture of three alkanes. Materials 2020, 13, 3689. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Hamilos, D.L. Rhinosinusitis diagnosis and management for the clinician: A synopsis of recent consensus guidelines. Mayo. Clin. Proc. 2011, 86, 427–443. [Google Scholar] [CrossRef] [Green Version]
- Merkus, P.; Ebbens, F.A.; Muller, B.; Fokkens, W.J. The ‘best method’ of topical nasal drug delivery: Comparison of seven techniques. Rhinology 2006, 44, 102–107. [Google Scholar]
- Cannady, S.B.; Batra, P.S.; Citardi, M.J.; Lanza, D.C. Comparison of delivery of topical medications to the paranasal sinuses via “vertex-to-floor” position and atomizer spray after FESS. Otolaryngol. Head Neck Surg. 2005, 133, 735–740. [Google Scholar] [CrossRef]
- Mori, E.; Merkonidis, C.; Cuevas, M.; Gudziol, V.; Matsuwaki, Y.; Hummel, T. The administration of nasal drops in the “Kaiteki” position allows for delivery of the drug to the olfactory cleft: A pilot study in healthy subjects. Eur. Arch. Otorhinolaryngol. 2016, 273, 939–943. [Google Scholar] [CrossRef]
- Masiuk, T.; Kadakia, P.; Wang, Z. Development of a physiologically relevant dripping analytical method using simulated nasal mucus for nasal spray formulation analysis. J. Pharm. Anal. 2016, 6, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Bonart, H.; Rajes, S.; Jung, J.; Repke, J.-U. Stability of gravity-driven liquid films overflowing microstructures with sharp corners. Phys. Rev. Fluids 2020, 5, 094001. [Google Scholar] [CrossRef]
- El Taoum, K.K.; Xi, J.; Kim, J.W.; Berlinski, A. In vitro evaluation of aerosols delivered via the nasal route. Respir Care 2015, 60, 1015–1025. [Google Scholar] [CrossRef] [Green Version]
- Doughty, D.V.; Hsu, W.; Dalby, R.N. Automated actuation of nasal spray products: Effect of hand-related variability on the in vitro performance of Flonase nasal spray. Drug Dev. Ind. Pharm. 2014, 40, 711–718. [Google Scholar] [CrossRef]
- Garcia, G.J.; Tewksbury, E.W.; Wong, B.A.; Kimbell, J.S. Interindividual variability in nasal filtration as a function of nasal cavity geometry. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Suman, J.D. In vitro anatomical models for nasal drug delivery. Pharmaceutics 2022, 14, 1353. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, M.; Xi, J.; Irshad, H.; Cheng, Y.-S. Nasal deposition in infants and children. J. Aerosol Med. 2014, 26, 110–116. [Google Scholar] [CrossRef]
- Berlinski, A. Pediatric aerosol therapy. Respir. Care 2017, 62, 662–677. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Xi, J.; Si, X.; Berlinski, A.; Su, W.C. Hood nebulization: Effects of head direction and breathing mode on particle inhalability and deposition in a 7-month-old infant model. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 209–218. [Google Scholar] [CrossRef]
- Lu, J.; Xi, J.; Langenderfer, J.E. Sensitivity analysis and uncertainty quantification in pulmonary drug delivery of orally inhaled pharmaceuticals. J. Pharm. Sci. 2017, 106, 3303–3315. [Google Scholar] [CrossRef]
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective—A review. Drug Deliv. Transl. Res. 2013, 3, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Wang, Z.; Si, X.A.; Zhou, Y. Nasal dilation effects on olfactory deposition in unilateral and bi-directional deliveries: In vitro tests and numerical modeling. Eur. J. Pharm. Sci. 2018, 118, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Far, J.; Abdel-Haq, M.; Gruber, M.; Abu Ammar, A. Developing Biodegradable Nanoparticles Loaded with Mometasone Furoate for Potential Nasal Drug Delivery. ACS Omega 2020, 5, 7432–7439. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seifelnasr, A.; Talaat, M.; Ramaswamy, P.; Si, X.A.; Xi, J. A Supine Position and Dual-Dose Applications Enhance Spray Dosing to the Posterior Nose: Paving the Way for Mucosal Immunization. Pharmaceutics 2023, 15, 359. https://doi.org/10.3390/pharmaceutics15020359
Seifelnasr A, Talaat M, Ramaswamy P, Si XA, Xi J. A Supine Position and Dual-Dose Applications Enhance Spray Dosing to the Posterior Nose: Paving the Way for Mucosal Immunization. Pharmaceutics. 2023; 15(2):359. https://doi.org/10.3390/pharmaceutics15020359
Chicago/Turabian StyleSeifelnasr, Amr, Mohamed Talaat, Pranav Ramaswamy, Xiuhua April Si, and Jinxiang Xi. 2023. "A Supine Position and Dual-Dose Applications Enhance Spray Dosing to the Posterior Nose: Paving the Way for Mucosal Immunization" Pharmaceutics 15, no. 2: 359. https://doi.org/10.3390/pharmaceutics15020359
APA StyleSeifelnasr, A., Talaat, M., Ramaswamy, P., Si, X. A., & Xi, J. (2023). A Supine Position and Dual-Dose Applications Enhance Spray Dosing to the Posterior Nose: Paving the Way for Mucosal Immunization. Pharmaceutics, 15(2), 359. https://doi.org/10.3390/pharmaceutics15020359