Current Status of Polysaccharides-Based Drug Delivery Systems for Nervous Tissue Injuries Repair
Abstract
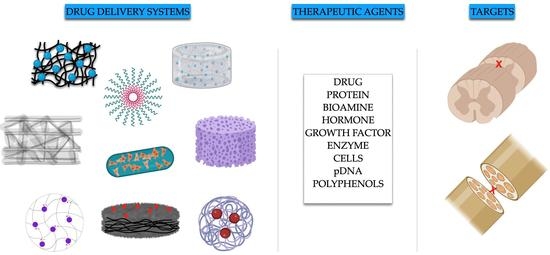
:1. Introduction
2. Chitosan (CS)
2.1. CS Associations
2.1.1. CS/HA Association
2.1.2. CS/ALG Association
3. ALGINATE
4. DEXTRAN
5. AGAROSE (AG)
6. CELLULOSE (CL)
7. GELLAN GUM (GG)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement:
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birch, R.; Birch, R.; Birch, R.; Birch, R. The Peripheral Nervous System: Anatomy and Function. In Peripheral Nerve Injuries: A Clinical Guide; Springer: London, UK, 2013; pp. 1–67. [Google Scholar]
- Thau, L.; Reddy, V.; Singh, P. Anatomy, Central Nervous System; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gautam, A. Afferent and Efferent Impulses. In Encyclopedia of Animal Cognition and Behavior; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–2. [Google Scholar]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Catala, M.; Kubis, N. Gross anatomy and development of the peripheral nervous system. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherland, 2013; Volume 115, pp. 29–41. [Google Scholar] [CrossRef]
- Rossi, S.; Vigani, B.; Sandri, G.; Bonferoni, M.C.; Ferrari, F. Design and criteria of electrospun fibrous scaffolds for the treatment of spinal cord injury. Neural Regen. Res. 2017, 12, 1786–1790. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic Spinal Cord Injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef]
- Li, R.; Li, D.-H.; Zhang, H.-Y.; Wang, J.; Li, X.-K.; Xiao, J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol. Sin. 2020, 41, 1289–1300. [Google Scholar] [CrossRef]
- Grinsell, D.; Keating, C.P. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. BioMed Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, F.; Arab, F.L.; Nikkhah, K.; Amiri, H.; Mahmoudi, M. Novel approaches using mesenchymal stem cells for curing peripheral nerve injuries. Life Sci. 2019, 221, 99–108. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Nanotechnology in peripheral nerve repair and reconstruction. Adv. Drug Deliv. Rev. 2019, 148, 308–343. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Reis, R.L.; Oliveira, J.M. Fundamentals and Current Strategies for Peripheral Nerve Repair and Regeneration. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; Volume 1249, pp. 173–201. [Google Scholar] [CrossRef]
- Sullivan, R.; Dailey, T.; Duncan, K.; Abel, N.; Borlongan, C.V. Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int. J. Mol. Sci. 2016, 17, 2101. [Google Scholar] [CrossRef]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. (Wars) 2011, 71, 281–299. [Google Scholar]
- Ramer, L.M.; Ramer, M.S.; Bradbury, E.J. Restoring function after spinal cord injury: Towards clinical translation of experimental strategies. Lancet Neurol. 2014, 13, 1241–1256. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef] [Green Version]
- Wieringa, P.; Pinho, A.R.; Micera, S.; A Van Wezel, R.J.; Moroni, L. Biomimetic Architectures for Peripheral Nerve Repair: A Review of Biofabrication Strategies. Adv. Health Mater. 2018, 7, e1701164. [Google Scholar] [CrossRef]
- Houshyar, S.; Bhattacharyya, A.; Shanks, R. Peripheral Nerve Conduit: Materials and Structures. ACS Chem. Neurosci. 2019, 10, 3349–3365. [Google Scholar] [CrossRef]
- Sarker; Naghieh, S.; McInnes, A.D.; Schreyer, D.J.; Chen, X. Strategic Design and Fabrication of Nerve Guidance Conduits for Peripheral Nerve Regeneration. Biotechnol. J. 2018, 13, e1700635. [Google Scholar] [CrossRef]
- Anjum, A.; Yazid, M.; Daud, M.F.; Idris, J.; Ng, A.; Naicker, A.S.; Ismail, O.; Kumar, R.A.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Arul, M.R.; Rudraiah, S.; Kalajzic, I.; Kumbar, S.G. Aligned microchannel polymer-nanotube composites for peripheral nerve regeneration: Small molecule drug delivery. J. Control. Release 2019, 296, 54–67. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, X.; Hu, H. Marine Polysaccharides as a Versatile Biomass for the Construction of Nano Drug De-livery Systems. Mar. Drugs 2021, 19, 345. [Google Scholar] [CrossRef]
- Rial-Hermida, M.I.; Rey-Rico, A.; Blanco-Fernandez, B.; Carballo-Pedrares, N.; Byrne, E.M.; Mano, J.F. Recent Progress on Polysaccharide-Based Hydrogels for Controlled Delivery of Therapeutic Biomolecules. ACS Biomater. Sci. Eng. 2021, 7, 4102–4127. [Google Scholar] [CrossRef]
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014, 25, 448–460. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Mehta, M.; Satija, S.; Aljabali, A.A.; Tambuwala, M.M.; Anand, K.; Sharma, N.; Dureja, H.; Jha, N.K.; et al. Current-status and applications of polysaccharides in drug delivery systems. Colloid Interface Sci. Commun. 2021, 42, 100418. [Google Scholar] [CrossRef]
- Chandrika, K.P.; Prasad, R.; Godbole, V. Development of chitosan-PEG blended films using Trichoderma: Enhancement of antimicrobial activity and seed quality. Int. J. Biol. Macromol. 2018, 126, 282–290. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, A.; Agrawal, G. Recent advances in polysaccharides based biomaterials for drug delivery and tissue engineering applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100067. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Boecker, A.; Daeschler, S.C.; Kneser, U.; Harhaus, L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front Cell Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef] [Green Version]
- Skop, N.B.; Calderon, F.; Levison, S.; Gandhi, C.D.; Cho, C.H. Heparin crosslinked chitosan microspheres for the delivery of neural stem cells and growth factors for central nervous system repair. Acta Biomater. 2013, 9, 6834–6843. [Google Scholar] [CrossRef]
- Wu, W.; Lee, S.-Y.; Wu, X.; Tyler, J.Y.; Wang, H.; Ouyang, Z.; Park, K.; Xu, X.-M.; Cheng, J.-X. Neuroprotective ferulic acid (FA)–glycol chitosan (GC) nanoparticles for functional restoration of traumatically injured spinal cord. Biomaterials 2014, 35, 2355–2364. [Google Scholar] [CrossRef] [Green Version]
- Gwak, S.-J.; Koo, H.; Yun, Y.; Yhee, J.Y.; Lee, H.Y.; Yoon, D.H.; Kim, K.; Ha, Y. Multifunctional nanoparticles for gene delivery and spinal cord injury. J. Biomed. Mater. Res. Part A 2015, 103, 3474–3482. [Google Scholar] [CrossRef]
- Ni, S.; Xia, T.; Li, X.; Zhu, X.; Qi, H.; Huang, S.; Wang, J. Sustained delivery of chondroitinase ABC by poly(propylene carbonate)-chitosan micron fibers promotes axon regeneration and functional recovery after spinal cord hemisection. Brain Res. 2015, 1624, 469–478. [Google Scholar] [CrossRef]
- Wang, D.; Wang, K.; Liu, Z.; Wang, Z.; Wu, H. Valproic acid-labeled chitosan nanoparticles promote recovery of neuronal injury after spinal cord injury. Aging (Albany NY) 2020, 12, 8953–8967. [Google Scholar] [CrossRef]
- Alizadeh, A.; Moradi, L.; Katebi, M.; Ai, J.; Azami, M.; Moradveisi, B.; Ostad, S.N. Delivery of injectable thermo-sensitive hydrogel releasing nerve growth factor for spinal cord regeneration in rat animal model. J. Tissue Viability 2020, 29, 359–366. [Google Scholar] [CrossRef]
- Kwiecien, J.M.; Zhang, L.; Yaron, J.R.; Schutz, L.N.; Kwiecien-Delaney, C.J.; Awo, E.A.; Burgin, M.; Dabrowski, W.; Lucas, A.R. Local Serpin Treatment via Chitosan-Collagen Hydrogel after Spinal Cord Injury Reduces Tissue Damage and Improves Neurologic Function. J. Clin. Med. 2020, 9, 1221. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Yu, H.; Song, Q.; Mao, P.; Li, K.; Bao, G. Sesamol-loaded stearic acid-chitosan nanomicelles mitigate the oxidative stress-stimulated apoptosis and induction of pro-inflammatory cytokines in motor neuronal of the spinal cord through NF-ĸB signaling pathway. Int. J. Biol. Macromol. 2021, 186, 23–32. [Google Scholar] [CrossRef]
- Javdani, M.; Ghorbani, R.; Hashemnia, M. Histopathological Evaluation of Spinal Cord with Experimental Traumatic Injury Following Implantation of a Controlled Released Drug Delivery System of Chitosan Hy-drogel Loaded with Selenium Nanoparticle. Biol. Trace Elem. Res. 2021, 199, 2677–2686. [Google Scholar] [CrossRef]
- Song, X.; Xu, Y.; Wu, J.; Shao, H.; Gao, J.; Feng, X.; Gu, J. A sandwich structured drug delivery composite membrane for improved recovery after spinal cord injury under longtime controlled release. Colloids Surf. B Biointerfaces 2020, 199, 111529. [Google Scholar] [CrossRef]
- Stenberg, L.; Kodama, A.; Lindwall-Blom, C.; Dahlin, L.B. Nerve regeneration in chitosan conduits and in autologous nerve grafts in healthy and in type 2 diabetic Goto-Kakizaki rats. Eur. J. Neurosci. 2015, 43, 463–473. [Google Scholar] [CrossRef]
- Li, X.; Wang, W.; Wei, G.; Wang, G.; Zhang, W.; Ma, X. Immunophilin FK506 loaded in chitosan guide promotes peripheral nerve regeneration. Biotechnol. Lett. 2010, 32, 1333–1337. [Google Scholar] [CrossRef]
- Farahpour, M.R.; Ghayour, S.J. Effect of in situ delivery of acetyl-L-carnitine on peripheral nerve regeneration and functional recovery in transected sciatic nerve in rat. Int. J. Surg. 2014, 12, 1409–1415. [Google Scholar] [CrossRef] [Green Version]
- Lopes, C.D.; Gonçalves, N.P.; Gomes, C.P.; Saraiva, M.J.; Pêgo, A.P. BDNF gene delivery mediated by neuron-targeted nanoparticles is neuroprotective in peripheral nerve injury. Biomaterials 2017, 121, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Jahromi, M.; Razavi, S.; Seyedebrahimi, R.; Reisi, P.; Kazemi, M. Regeneration of Rat Sciatic Nerve Using PLGA Conduit Containing Rat ADSCs with Controlled Release of BDNF and Gold Nanoparticles. J. Mol. Neurosci. 2020, 71, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Hui, H.; Liu, Z.; Chang, Z.; Wang, M.; He, B.; Hao, D. TPP ionically cross-linked chitosan/PLGA microspheres for the delivery of NGF for peripheral nerve system repair. Carbohydr. Polym. 2021, 258, 117684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Y.; Xu, H.; Bao, Y.; Yan, X.; Li, Y.; Li, Y.; Yin, Y.; Wang, X.; Qiu, T.; et al. Preparation and evaluation of an injectable chitosan-hyaluronic acid hydrogel for peripheral nerve regeneration. J. Wuhan Univ. Technol. Sci. Ed. 2016, 31, 1401–1407. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.; Bao, Y.; Yan, X.; Yin, Y.; Li, Y.; Wang, X.; Huang, Z.; Xu, P. Preparation and characterization of injectable chitosan–hyaluronic acid hydrogels for nerve growth factor sustained release. J. Bioact. Compat. Polym. 2016, 32, 146–162. [Google Scholar] [CrossRef]
- Han, G.H.; Kim, S.J.; Ko, W.-K.; Lee, D.; Lee, J.S.; Nah, H.; Han, I.-B.; Sohn, S. Injectable Hydrogel Containing Tauroursodeoxycholic Acid for Anti-neuroinflammatory Therapy After Spinal Cord Injury in Rats. Mol. Neurobiol. 2020, 57, 4007–4017. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Rui, M.; Collina, S.; Fagiani, F.; Lanni, C.; Ferrari, F. Dual-Functioning Scaffolds for the Treatment of Spinal Cord Injury: Alginate Nanofibers Loaded with the Sigma 1 Receptor (S1R) Agonist RC-33 in Chitosan Films. Mar. Drugs 2019, 18, 21. [Google Scholar] [CrossRef] [Green Version]
- Rahmati, M.; Ehterami, A.; Saberani, R.; Abbaszadeh-Goudarzi, G.; Kolarijani, N.R.; Khastar, H.; Garmabi, B.; Salehi, M. Improving sciatic nerve regeneration by using alginate/chitosan hydrogel containing berberine. Drug Deliv. Transl. Res. 2020, 11, 1983–1993. [Google Scholar] [CrossRef]
- Ashraf, R.; Sofi, H.S.; Beigh, M.A.; Majeed, S.; Arjamand, S.; Sheikh, F.A. Prospects of Natural Polymeric Scaffolds in Peripheral Nerve Tissue-Regeneration. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2018; Volume 1077, pp. 501–525. [Google Scholar] [CrossRef]
- Grijalvo, S.; Nieto-Díaz, M.; Maza, R.M.; Eritja, R.; Díaz, D.D. Alginate Hydrogels as Scaffolds and Delivery Systems to Repair the Damaged Spinal Cord. Biotechnol. J. 2019, 14, 1900275. [Google Scholar] [CrossRef] [Green Version]
- Downing, T.L.; Wang, A.; Yan, Z.-Q.; Nout, Y.; Lee, A.L.; Beattie, M.S.; Bresnahan, J.C.; Farmer, D.L.; Li, S. Drug-eluting microfibrous patches for the local delivery of rolipram in spinal cord repair. J. Control. Release 2012, 161, 910–917. [Google Scholar] [CrossRef] [Green Version]
- Ansorena, E.; De Berdt, P.; Ucakar, B.; Simón-Yarza, T.; Jacobs, D.; Schakman, O.; Jankovski, A.; Deumens, R.; Blanco-Prieto, M.J.; Préat, V.; et al. Injectable alginate hydrogel loaded with GDNF promotes functional recovery in a hemisection model of spinal cord injury. Int. J. Pharm. 2013, 455, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Des Rieux, A.; De Berdt, P.; Ansorena, E.; Ucakar, B.; Damien, J.; Schakman, O.; Audouard, E.; Bouzin, C.; Auhl, D.; Simón-Yarza, T.; et al. Vascular endothelial growth factor-loaded injectable hydrogel enhances plasticity in the injured spinal cord: Vegf-Loaded Injectable Hydrogel. J. Biomed. Mater. Res. Part A 2014, 102, 2345–2355. [Google Scholar] [CrossRef]
- Nazemi, Z.; Nourbakhsh, M.S.; Kiani, S.; Heydari, Y.; Ashtiani, M.K.; Daemi, H.; Baharvand, H. Co-delivery of minocycline and paclitaxel from injectable hydrogel for treatment of spinal cord injury. J. Control. Release 2020, 321, 145–158. [Google Scholar] [CrossRef]
- Caterina, V.; Barbara, V.; Ilaria, F.; Dalila, M.; Giorgio, M.; Lorenzo, M.; Ferrari, F.; Giuseppina, S.; Silvia, R. Development of alginate-spermidine micro/nanogels as potential antioxidant and anti-inflammatory tool in peripheral nerve injuries. Formul. Stud. Phys. Chem. Characterization. Int. J. Pharm. 2022, 626, 122168. [Google Scholar] [CrossRef]
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999. [Google Scholar] [CrossRef]
- Wasiak, I.; Kulikowska, A.; Janczewska, M.; Michalak, M.; Cymerman, I.A.; Nagalski, A.; Kallinger, P.; Szymanski, W.W.; Ciach, T. Dextran Nanoparticle Synthesis and Properties. PLoS ONE 2016, 11, e0146237. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Jiang, H.; Cui, X.; Liang, G.; Gao, M.; Huang, Z.; Xi, Q. Synthesis of methylprednisolone loaded ibuprofen modified dextran based nanoparticles and their application for drug delivery in acute spinal cord injury. Oncotarget 2017, 8, 99666–99680. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Quan, P.; Li, Q.; Tang, P.; Chen, J.; Jiang, T.; Cai, W. Dextran-based biodegradable nanoparticles: An alternative and convenient strategy for treatment of traumatic spinal cord injury. Int. J. Nanomed. 2018, 13, 4121–4132. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chen, J.; Zhao, S.; Huang, T.; Ying, H.; Trujillo, C.; Molinaro, G.; Zhou, Z.; Jiang, T.; Li, L.; et al. High drug-loaded microspheres enabled by controlled in-droplet precipitation promote functional recovery after spinal cord injury. Nat. Commun. 2022, 13, 1262. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Aga-rose-Based Biomaterials for Tissue Engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Azarfam, M.Y.; Nasirinezhad, M.; Naeim, H.; Zarrintaj, P.; Saeb, M. A Green Composite Based on Gelatin/Agarose/Zeolite as a Potential Scaffold for Tissue Engineering Applications. J. Compos. Sci. 2021, 5, 125. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Taghizadeh, A.; Taghizadeh, M.; Stadler, F.J.; Farokhi, M.; Mottaghitalab, F.; Zarrintaj, P.; Ramsey, J.D.; Seidi, F.; Saeb, M.R.; et al. Agarose-based biomaterials for advanced drug delivery. J. Control. Release 2020, 326, 523–543. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kim, Y.-T.; McKeon, R.J.; Bellamkonda, R.V. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials 2006, 27, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; McKeon, R.J.; Brady-Kalnay, S.M.; Bellamkonda, R.V. Sustained Delivery of Activated Rho GTPases and BDNF Promotes Axon Growth in CSPG-Rich Regions Following Spinal Cord Injury. PLoS ONE 2011, 6, e16135. [Google Scholar] [CrossRef] [Green Version]
- Chvatal, S.A.; Kim, Y.-T.; Bratt-Leal, A.M.; Lee, H.; Bellamkonda, R.V. Spatial distribution and acute anti-inflammatory effects of Methylprednisolone after sustained local delivery to the contused spinal cord. Biomaterials 2008, 29, 1967–1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; McKeon, R.J.; Bellamkonda, R.V. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2009, 107, 3340–3345. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Lu, P.; Bednark, B.; Lynam, D.; Conner, J.M.; Sakamoto, J.; Tuszynski, M.H. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials 2013, 34, 1529–1536. [Google Scholar] [CrossRef] [Green Version]
- Cox, A.; Varma, A.; Barry, J.; Vertegel, A.; Banik, N. Nanoparticle Estrogen in Rat Spinal Cord Injury Elicits Rapid Anti-Inflammatory Effects in Plasma, Cerebrospinal Fluid, and Tissue. J. Neurotrauma 2015, 32, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Shultz, R.B.; Wang, Z.; Nong, J.; Zhang, Z.; Zhong, Y. Local delivery of thyroid hormone enhances oligodendrogenesis and myelination after spinal cord injury. J. Neural Eng. 2017, 14, 036014. [Google Scholar] [CrossRef]
- Wang, Z.; Nong, J.; Shultz, R.B.; Zhang, Z.; Kim, T.; Tom, V.J.; Ponnappan, R.K.; Zhong, Y. Local delivery of minocycline from metal ion-assisted self-assembled complexes promotes neuroprotection and functional recovery after spinal cord injury. Biomaterials 2017, 112, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, B.; Wang, Z.; Nong, J.; Urban, M.W.; Zhang, Z.; Trovillion, V.A.; Wright, M.C.; Zhong, Y.; Lepore, A.C. Local BDNF delivery to the injured cervical spinal cord using an engineered hydrogel enhances diaphragmatic respiratory function. J. Neurosci. 2018, 38, 5982–5995. [Google Scholar] [CrossRef]
- Gao, M.; Lu, P.; Lynam, D.; Bednark, B.; Campana, W.M.; Sakamoto, J.; Tuszynski, M. BDNF gene delivery within and beyond templated agarose multi-channel guidance scaffolds enhances peripheral nerve regeneration. J. Neural Eng. 2016, 13, 066011. [Google Scholar] [CrossRef]
- Dutta, S.D.; Patel, D.K.; Lim, K.-T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Jabbari, F.; Babaeipour, V.; Bakhtiari, S. Bacterial cellulose-based composites for nerve tissue engineering. Int. J. Biol. Macromol. 2022, 217, 120–130. [Google Scholar] [CrossRef]
- Stumpf, T.R.; Tang, L.; Kirkwood, K.; Yang, X.; Zhang, J.; Cao, X. Production and evaluation of biosynthesized cellulose tubes as promising nerve guides for spinal cord injury treatment. J. Biomed. Mater. Res. Part A 2020, 108, 1380–1389. [Google Scholar] [CrossRef]
- Luo, L.; Gan, L.; Liu, Y.; Tian, W.; Tong, Z.; Wang, X.; Huselstein, C.; Chen, Y. Construction of nerve guide conduits from cellulose/soy protein composite membranes combined with Schwann cells and pyrroloquinoline quinone for the repair of peripheral nerve defect. Biochem. Biophys. Res. Commun. 2015, 457, 507–513. [Google Scholar] [CrossRef]
- Muthukumar, T.; Song, J.E.; Khang, G. Biological Role of Gellan Gum in Improving Scaffold Drug Delivery, Cell Adhesion Properties for Tissue Engineering Applications. Molecules 2019, 24, 4514. [Google Scholar] [CrossRef] [Green Version]
- Kirchmajer, D.M.; Steinhoff, B.; Warren, H.; Clark, R.; Panhuis, M.I.H. Enhanced gelation properties of purified gellan gum. Carbohydr. Res. 2014, 388, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Stevens, L.R.; Gilmore, K.J.; Wallace, G.G.; Panhuis, M.I.H. Tissue engineering with gellan gum. Biomater. Sci. 2016, 4, 1276–1290. [Google Scholar] [CrossRef] [Green Version]
- Sweety, J.P.; Selvasudha, N.; Dhanalekshmi, U.M.; Sridurgadevi, N. Gellan Gum and Its Composites: Suitable Candidate for Efficient Nanodrug Delivery. In Nanoengineering of Biomaterials; Wiley: Hoboken, NJ, USA, 2021; pp. 33–61. [Google Scholar] [CrossRef]
- Vigani, B.; Valentino, C.; Cavalloro, V.; Catenacci, L.; Sorrenti, M.; Sandri, G.; Bonferoni, M.; Bozzi, C.; Collina, S.; Rossi, S.; et al. Gellan-based composite system as a potential tool for the treatment of nervous tissue injuries: Cross-linked electrospun nanofibers embedded in a rc-33-loaded freeze-dried matrix. Pharmaceutics 2021, 13, 164. [Google Scholar] [CrossRef]
- Vigani, B.; Valentino, C.; Sandri, G.; Caramella, C.M.; Ferrari, F.; Rossi, S. Spermidine crosslinked gellan gum-based “hydrogel nanofibers” as potential tool for the treatment of nervous tissue injuries: A formulation study. Int. J. Nanomed. 2022, 17, 3421–3439. [Google Scholar] [CrossRef] [PubMed]
- Ramburrun, P.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. Design and characterization of neurodurable gellan-xanthan pH-responsive hydrogels for controlled drug delivery. Expert Opin. Drug Deliv. 2016, 14, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, A.; Zhong, Y.; Huang, L.; Yang, J.; Zhou, C.; Zhou, L.; Zhang, Y.; Fu, G. Laminin-modified gellan gum hydrogels loaded with the nerve growth factor to enhance the proliferation and differentiation of neuronal stem cells. RSC Adv. 2020, 10, 17114–17122. [Google Scholar] [CrossRef] [PubMed]







| DDS | Production Technique | Therapeutic Agent | Application | In Vitro Model | In Vivo Model | Reference |
|---|---|---|---|---|---|---|
| CS microspheres | Genepin-cross-linking and coaxial airflow technique | Heparin | SCI | Neural stem cell line | - | [32] |
| Glycol/CS Nps | Self-assembling | Ferulic acid | SCI | Primary spinal cord neurons culture | Rat spinal cord contusion injury model | [33] |
| CS Nps | Ionotropic gelation | Methylprednisolone | SCI | Mouse neural stem cells | Compressed spinal cord injury rat model | [34] |
| Microfibers containinigChABC-loaded CS microparticles | Ionotropic gelation (with TPP)and electrospinning | Chondroitinase ABC | SCI | - | Hemisected thoracic rad spinal cord model | [35] |
| CS Nps | conjugating by coupling carboxyl to amino group in the presence of modification reagents and dyalization to isolate the conjugates | Valproic acid | SCI | - | Spinal cord contusion rat injury model | [36] |
| CS-based thermo-sensitive hydrogel | Hydroxyethylcellulose as cross-linking agent, β-glycerol phosphate disodium salt pentahydrate as gelling agent for CS solution | Lentiviral mediated NGF–overexpressing hADSCs | SCI | - | Contusive rat spinal cord injury model | [37] |
| CS-collagen based hydrogel loaded with Serp-1 | Lyophilization | Serpine (Serp-1) | SCI | - | Dorsal column crush rat model | [38] |
| CS-stearic acid conjugated nanomicelles loaded with sesamol | Centrifugation followed by freeze-drying | Sesamol | SCI | NSC-34 cell line | - | [39] |
| CS Hydrogel loaded with Nps | Addition of sodium hydroxide | Selenium | SCI | - | Aneurysm clamping at the level of thoracic vertebrae | [40] |
| Sandwich system: PLA fibers; NGF-loaded PLGA- microspheres-CS fibers | Electrospinning (PLA fibers and CS fibers), ultrasonication and electrospraying (PLGA microspheres) | NGF | SCI | PC-12 cell line | Allen’s SCI models on rats | [41] |
| CS tubular conduit | Solvent casting in tubular mold and subsequent immersion in NaOH | FK506 | PNI | - | Sciatic nerve injury rat model | [43] |
| CS/glycerol tubular conduit | Home-made tubular mold | Acetyl-L-carnitine | PNI | - | Left sciatic nerve transection on rats | [44] |
| TMCSH-HC Nps | Mixing of TMCSH and pDNA, lyophilization and addition of HC. | pDNA encoding for BDNF | PNI | - | Injection of nanoparticles before nerve crush injury induction | |
| CS Nerve guide conduit with aligned microchannels loaded with halloysite nanotubes | Unidirectional freezing in N2 and freeze-drying, cross-linking with epichlorohydrin | Aminopyridine | PNI | Schwann cell line | Sciatic nerve defect rat model | [22] |
| PLLA nanotubes containing fibrin hydrogel loaded with curcumin encapsulated CS Nps and SCs | Electrospinning (PLLA nanotubes) | Curcumin | PNI | Schwann cells | Sciatic nerve injury rat model | [46] |
| CS/PLGA microspheres | Re-emulsification TPP ionic cross-linking method | NGF | PNI | PC12 cells | Sciatic nerve injury rat model | [47] |
| Associations | ||||||
| Oxidized HA/glycol CS hydrogel | Cross-linking and freeze-drying | tauroursodeoxycholic acid | SCI | - | Mechanical SCI rat model | [50] |
| HA/CS injectable hydrogel | Prepared at 37 °C, using ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) and freeze-drying | NGF | PNI | BMMSCs | - | [48] |
| RC-33 loaded-ALG nanofibers embedded in CS film | Electrospinning (nanofibers), solvent casting (film) | RC-33 | SCI | SH-SY5Y cells | - | [51] |
| Berberine-loaded ALG/CShydrogel | CaCl2 cross-linked ALG added dropwise to CS solution containing β-glycerol phosphate | berberine | PNI | PC12 cells | crush-induced sciatic nerve rat model | [52] |
| DDS | Production Technique | Therapeutic Agent | Application | In Vitro Model | In Vivo Model | Reference |
|---|---|---|---|---|---|---|
| PLA-based microfibers coated with a CaCl2- cross-linked ALG hydrogel layer | Electrospinning (microfibers), cross-linking (hydrogel) | Rolipram | SCI | - | Rats subjected to C5 hemisection lesion | [55] |
| CaCl2 cross-linked ALG- fibrinogen hydrogel embedded with PLGA microspheres | Solvent extraction/evaporation (microspheres) | GDNF | SCI | PC-12 cells | Rat spinal cord hemisection model | [56] |
| CaCl2 cross-linked ALG: fibrinogen-based hydrogel containing loaded CS-dextran sulfate Nps or PLGA microspheres | CaCl2 cross-linking (hydrogel); not reported for microspheres and Nps | VEGF | SCI | SH-SY5Y and NIH-3T3 cells | Rat spinal cord hemisection model | [57] |
| MH ALG or ALG-S complex; PLGA-based microspheres embedded in an ALG or ALG-S hydrogel | Lyophilization for Hydrogel; single (oil/water) emulsion/solvent evaporation method for microspheres | MH and PCX | SCI | - | Left lateral hemisection animal (rats) model | [58] |
| ALG-spermidine cross-linked hydrogel | Ionotropic gelation and freeze-drying | Spermidine | PNI | Schwann cells | - | [59] |
| DDS | Production Technique | Therapeutic Agent | Application | In Vitro Model | In Vivo Model | Reference |
|---|---|---|---|---|---|---|
| Ibuprofen-Dx Nps | Esterification between the hydroxyl groups of Dx and the carboxylic acid groups of ibuprofen, activated with N, N-carbonyldiimidazole. | Methylprednisolone | SCI | BV-12 microglial cells | Intraperitoneal injection in an SCI rat model | [62] |
| Acetalated-Dx Nps | Microprecipitation method | PCX | SCI | - | Mechanical SCI rat model | [63] |
| Acetalated-Dx Nps nano-in-micro structured microspheres | Microfluidic flow-focusing device | Methylprednisolone | SCI | - | Mechanical SCI rat model | [64] |
| DDS | Production Technique | Therapeutic Agent | Application | In Vitro Model | In Vivo Model | Reference |
|---|---|---|---|---|---|---|
| Loaded-lipid microtubes embedded within AG injectable hydrogel | Self-assembling of lipid microtubes, then added to AG solution. | BNDF | SCI | - | Dorsal over-hemisection rat model | [68] |
| Loaded-lipid microtubes embedded within AG injectable hydrogel | Self-assembling of lipid microtubes, then added to AG solution | BNDF, CA-Cdc42 and CA-Rac1 | SCI | - | Modified dorsal-over hemisection rat model | [69] |
| AG hydrogel containing loaded-PLGA Nps | Double emulsion method (Nps) | PCX | SCI | - | Mechanical SCI rat model | [70] |
| Loaded-lipid microtubes embedded in an AG-based hydrogel | Thermal stabilization of chondroitinase ABC with trehalose | Chondroitinase ABC | SCI | - | Dorsal-over-hemisection injury | [71] |
| AG scaffold with hexagonally packed multi-channel guides | Multi-component fiber bundle templates (channels diameter: 166 μm) | Syngeneic marrow stromal cells expressing BDNF | SCI | - | Complete transection of rat severe injury model | [72] |
| AG hydrogel dispersed with loaded PLGA Nps | Nanoprecipitation method and lyophilization (Nps) | Estrogen (E2) | SCI | - | Moderate to severe SCI rat model | [73] |
| Loaded-AG hydrogel | AG prepared in artificial cerebrospinal fluid and then added with T3 as insoluble particles (obtained neutralizing) | Thyroid hormone 3,3′,5-triiodothyronine (T3). | SCI | - | Unilateral cervical spinal cord contusion injury rat model | [74] |
| AG containing loaded-Dx sulfate complex | Metal ion-assisted interaction | MH | SCI | - | Unilateral cervical spinal cord contusion injury rat model | [75] |
| AG embedded with loaded- Dx-CS- particles | Self-assembling by electrostatic interactions (particles) | BNDF | SCI | - | Unilateral cervical spinal cord contusion injury rat model | [76] |
| AG scaffold with hexagonally packed multi-channel guides | Multi-component fiber bundle templates (channels diameter of 200 μm) | BNDF | SCI | - | Sciatic nerve gaps rat model | [77] |
| DDS | Production Technique | Therapeutic Agent | Application | In Vitro Model | In Vivo Model | Reference |
|---|---|---|---|---|---|---|
| Biosynthesized cellulose (BC) tubes | Custom-designed bioreactor with silicon tube as mold | NGF | SCI | PC12 cells | - | [81] |
| CL-soy protein tubes seeded with SC cells | Tubular mold | Pyrroloquinolinequinone (PQQ) | SCI | - | Sciatic nerve injury rat model | [82] |
| DDS | Production Technique | Therapeutic Agent | Application | In Vitro Model | In Vivo Model | Reference |
|---|---|---|---|---|---|---|
| CaCl2-cross-linked GG nanofibers embedded with loaded-GG freeze-dried matrix, | Electrospinning (nanofibers), freeze-drying (porous matrix) | RC-33 | SCI | - | - | [87] |
| hydrogel conduits of GG and xanthan gum intercalated with polymethyl methacrylate particles | Thermal-ionic cross-linking mechanism | Bovine serum albumin (BSA) and diclofenac sodium | PNI | - | - | [89] |
| thiolated GG hydrogel | Prepared at 60 °C and then poured into a mold to form in situ gel | Laminin and NGF | PNI | Rat neural stem cell | - | [90] |
| GG/GL nanofibers | Electrospinning | Spermidine | SCI/PNI | Schwann cells | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentino, C.; Vigani, B.; Sandri, G.; Ferrari, F.; Rossi, S. Current Status of Polysaccharides-Based Drug Delivery Systems for Nervous Tissue Injuries Repair. Pharmaceutics 2023, 15, 400. https://doi.org/10.3390/pharmaceutics15020400
Valentino C, Vigani B, Sandri G, Ferrari F, Rossi S. Current Status of Polysaccharides-Based Drug Delivery Systems for Nervous Tissue Injuries Repair. Pharmaceutics. 2023; 15(2):400. https://doi.org/10.3390/pharmaceutics15020400
Chicago/Turabian StyleValentino, Caterina, Barbara Vigani, Giuseppina Sandri, Franca Ferrari, and Silvia Rossi. 2023. "Current Status of Polysaccharides-Based Drug Delivery Systems for Nervous Tissue Injuries Repair" Pharmaceutics 15, no. 2: 400. https://doi.org/10.3390/pharmaceutics15020400
APA StyleValentino, C., Vigani, B., Sandri, G., Ferrari, F., & Rossi, S. (2023). Current Status of Polysaccharides-Based Drug Delivery Systems for Nervous Tissue Injuries Repair. Pharmaceutics, 15(2), 400. https://doi.org/10.3390/pharmaceutics15020400