One-Step Phytofabrication Method of Silver and Gold Nanoparticles Using Haloxylon salicornicum for Anticancer, Antimicrobial, and Antioxidant Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
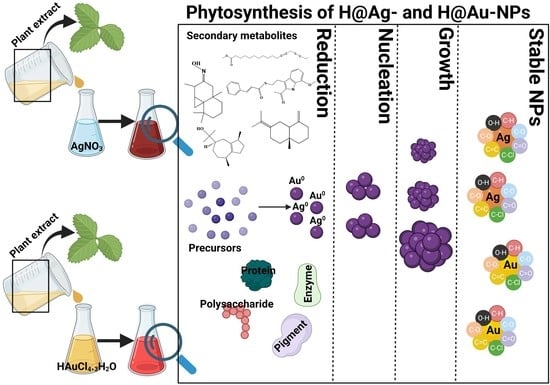
2.2.1. Preparation of Plant Extracts
2.2.2. Gas Chromatography-Mass Spectroscopy
2.2.3. Silver and Gold Nanoparticle Synthesis Using Aqueous Plant Extracts
2.2.4. Physicochemical Characterization of Silver and Gold Nanoparticles
UV-Vis Spectroscopy
X-ray Diffraction Analysis
Fourier-Transform Infrared Spectroscopy
Transmission Electron Microscope
Scanning Electron Microscope, Energy-Dispersive X-ray, and Mapping Analysis
Dynamic Light Scattering and Zeta Potential Analysis
2.2.5. Antimicrobial Activity of Silver Nanoparticles
Agar Well Diffusion Method
Minimum Inhibition and Maximum Bactericidal Concentrations
2.2.6. Anticancer Activity of Silver Nanoparticles
Cell Culture
MTT Assay
2.2.7. Antioxidant Activity of Nanoparticles
DPPH Assay
FRAP Assay
2.2.8. Statistical Analysis
3. Results
3.1. GC-MS Analysis of Haloxylon Salicornicum
3.2. Ag-NP and Au-NP Synthesis
3.2.1. XRD Analysis
3.2.2. FTIR
3.2.3. Transmission Electron Microscope
3.2.4. Scanning Electron Microscope and EDX
3.2.5. Zeta Potential and DLS Analysis
3.3. Anticancer Activity of Ag-NPs
3.4. Antimicrobial Activity of Ag-NPs
3.5. Antioxidant Activity of Ag-NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dauthal, P.; Mukhopadhyay, M. Noble metal nanoparticles: Plant-mediated synthesis, mechanistic aspects of synthesis, and applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Khan, F.; Shariq, M.; Asif, M.; Siddiqui, M.A.; Malan, P.; Ahmad, F. Green nanotechnology: Plant-mediated nanoparticle synthesis and application. Nanomaterials 2022, 12, 673. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.S.; Álvarez-López, V.; Becerra-Castro, C.; Cabello-Conejo, M.; Prieto-Fernández, Á. Potential role of plant-associated bacteria in plant metal uptake and implications in phytotechnologies. Adv. Bot. Res. 2017, 83, 87–126. [Google Scholar]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, Y.; He, Z.; Stoffella, P.J. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 339–353. [Google Scholar] [CrossRef]
- Bisht, S.; Sharma, V.; Kumari, N. Biosynthesized magnetite nanoparticles from Polyalthia longifolia leaves improve photosynthetic performance and yield of Trigonella foenum-graecum under drought stress. Plant Stress 2022, 5, 100090. [Google Scholar] [CrossRef]
- Prabha, S.; Durgalakshmi, D.; Rajendran, S.; Lichtfouse, E. Plant-derived silica nanoparticles and composites for biosensors, bioimaging, drug delivery and supercapacitors: A review. Environ. Chem. Lett. 2021, 19, 1667–1691. [Google Scholar] [CrossRef]
- de Morais, M.G.; Vaz, B.d.S.; de Morais, E.G.; Costa, J.A.V. Biologically active metabolites synthesized by microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef]
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of biogenic capping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Front. Nanotechnol. 2022, 3, 105. [Google Scholar] [CrossRef]
- Chugh, R.; Kaur, G. A mini review on green synthesis of nanoparticles by utilization of Musa-balbisiana waste peel extract. Mater. Today Proc. 2022. [Google Scholar] [CrossRef]
- Manousaki, A.; Jancheva, M.; Grigorakis, S.; Makris, D.P. Extraction of antioxidant phenolics from agri-food waste biomass using a newly designed glycerol-based natural low-transition temperature mixture: A comparison with conventional eco-friendly solvents. Recycling 2016, 1, 194–204. [Google Scholar] [CrossRef]
- Chung, I.-M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-mediated synthesis of silver nanoparticles: Their characteristic properties and therapeutic applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Skóra, B.; Krajewska, U.; Nowak, A.; Dziedzic, A.; Barylyak, A.; Kus-Liśkiewicz, M. Noncytotoxic silver nanoparticles as a new antimicrobial strategy. Sci. Rep. 2021, 11, 13451. [Google Scholar] [CrossRef] [PubMed]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef]
- Si, P.; Razmi, N.; Nur, O.; Solanki, S.; Pandey, C.M.; Gupta, R.K.; Malhotra, B.D.; Willander, M.; de la Zerda, A. Gold nanomaterials for optical biosensing and bioimaging. Nanoscale Adv. 2021, 3, 2679–2698. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef]
- Li, F.-Y.; Chung, Y.-C. Fabrication of “electroactive cells” using bio-inspired polydopamine-derived carbon nanoparticles for manipulation of cells with electrical stimulation. Front. Bioeng. Biotechnol. 2022, 10, 949308. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Panwar, J.; Yun, Y.-S. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Wang, L.; Hasanzadeh Kafshgari, M.; Meunier, M. Optical properties and applications of plasmonic-metal nanoparticles. Adv. Funct. Mater. 2020, 30, 2005400. [Google Scholar] [CrossRef]
- Lomelí-Rosales, D.A.; Zamudio-Ojeda, A.; Reyes-Maldonado, O.K.; López-Reyes, M.E.; Basulto-Padilla, G.C.; Lopez-Naranjo, E.J.; Zuñiga-Mayo, V.M.; Velázquez-Juárez, G. Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Capsicum chinense Plant. Molecules 2022, 27, 1692. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Devanesan, S.; Alfuraydi, A.A.; Vishnubalaji, R.; Munusamy, M.A.; Murugan, K.; Nicoletti, M.; Benelli, G. Green synthesis of silver nanoparticles using Pimpinella anisum seeds: Antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. Int. J. Nanomed. 2016, 11, 4439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devanesan, S.; AlSalhi, M.S. Green synthesis of silver nanoparticles using the flower extract of Abelmoschus esculentus for cytotoxicity and antimicrobial studies. Int. J. Nanomed. 2021, 16, 3343. [Google Scholar] [CrossRef] [PubMed]
- Hosny, M.; Fawzy, M.; El-Fakharany, E.M.; Omer, A.M.; Abd El-Monaem, E.M.; Khalifa, R.E.; Eltaweil, A.S. Biogenic synthesis, characterization, antimicrobial, antioxidant, antidiabetic, and catalytic applications of platinum nanoparticles synthesized from Polygonum salicifolium leaves. J. Environ. Chem. Eng. 2022, 10, 106806. [Google Scholar] [CrossRef]
- Liang, Y.; Demir, H.; Wu, Y.; Aygun, A.; Tiri, R.N.E.; Gur, T.; Yuan, Y.; Xia, C.; Demir, C.; Sen, F. Facile synthesis of biogenic palladium nanoparticles using biomass strategy and application as photocatalyst degradation for textile dye pollutants and their in-vitro antimicrobial activity. Chemosphere 2022, 306, 135518. [Google Scholar] [CrossRef]
- Ansari, A.; Siddiqui, V.U.; Rehman, W.U.; Akram, M.K.; Siddiqi, W.A.; Alosaimi, A.M.; Hussein, M.A.; Rafatullah, M. Green synthesis of TiO2 nanoparticles using Acorus calamus leaf extract and evaluating its photocatalytic and in vitro antimicrobial activity. Catalysts 2022, 12, 181. [Google Scholar] [CrossRef]
- Haydar, M.S.; Das, D.; Ghosh, S.; Mandal, P. Implementation of mature tea leaves extract in bioinspired synthesis of iron oxide nanoparticles: Preparation, process optimization, characterization, and assessment of therapeutic potential. Chem. Pap. 2022, 76, 491–514. [Google Scholar] [CrossRef]
- Vinothkanna, A.; Mathivanan, K.; Ananth, S.; Ma, Y.; Sekar, S. Biosynthesis of copper oxide nanoparticles using Rubia cordifolia bark extract: Characterization, antibacterial, antioxidant, larvicidal and photocatalytic activities. In Environmental Science and Pollution Research; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–12. [Google Scholar]
- Mary, J.V.; Pragathiswaran, C.; Anusuya, N. Photocatalytic, degradation, sensing of Pb2+ using titanium nanoparticles synthesized via plant extract of Cissusquadrangularis: In-vitroanalysis of microbial and anti-cancer activities. J. Mol. Struct. 2021, 1236, 130144. [Google Scholar] [CrossRef]
- Mortazavi-Derazkola, S.; Hosseinzadeh, M.; Yousefinia, A.; Naghizadeh, A. Green Synthesis and Investigation of Antibacterial Activity of Silver Nanoparticles Using Eryngium bungei Boiss Plant Extract. J. Polym. Environ. 2021, 29, 2978–2985. [Google Scholar] [CrossRef]
- Happy, A.; Soumya, M.; Kumar, S.V.; Rajeshkumar, S.; Sheba, R.D.; Lakshmi, T.; Nallaswamy, V.D. Phyto-assisted synthesis of zinc oxide nanoparticles using Cassia alata and its antibacterial activity against Escherichia coli. Biochem. Biophys. Rep. 2019, 17, 208–211. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Kwon, D.-N.; Kim, J.-H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res. Lett. 2014, 9, 373. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Redhwan, A.; Bin-Meferij, M.M. Cyanobacteria–A Promising Platform in Green Nanotechnology: A Review on Nanoparticles Fabrication and Their Prospective Applications. Int. J. Nanomed. 2020, 15, 6033–6066. [Google Scholar] [CrossRef]
- Nagajyothi, P.; Cha, S.J.; Yang, I.J.; Sreekanth, T.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Saratale, G.D.; Ahn, S.; Shin, H.-S. Grape pomace extracted tannin for green synthesis of silver nanoparticles: Assessment of their antidiabetic, antioxidant potential and antimicrobial activity. Polymers 2021, 13, 4355. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, S.; Rahuman, A.A.; Rajakumar, G.; Santhoshkumar, T.; Kirthi, A.V.; Jayaseelan, C.; Bagavan, A.; Zahir, A.A.; Elango, G.; Kamaraj, C. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol. Res. 2011, 108, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Thakar, M.A.; Jha, S.S.; Phasinam, K.; Manne, R.; Qureshi, Y.; Babu, V.H. X ray diffraction (XRD) analysis and evaluation of antioxidant activity of copper oxide nanoparticles synthesized from leaf extract of Cissus vitiginea. Mater. Today Proc. 2022, 51, 319–324. [Google Scholar] [CrossRef]
- Aygun, A.; Gulbagca, F.; Altuner, E.E.; Bekmezci, M.; Gur, T.; Karimi-Maleh, H.; Karimi, F.; Vasseghian, Y.; Sen, F. Highly active PdPt bimetallic nanoparticles synthesized by one-step bioreduction method: Characterizations, anticancer, antibacterial activities and evaluation of their catalytic effect for hydrogen generation. Int. J. Hydrog. Energy 2022, 48, 6666–6679. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in clinical translation for cancer therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalil, M.I.; Al-Zaban, M.I. Novel Biogenic Silver Nanoparticle-Induced Reactive Oxygen Species Inhibit the Biofilm Formation and Virulence Activities of Methicillin-Resistant Staphylococcus aureus (MRSA) Strain. Front. Bioeng. Biotechnol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Jebril, S.; Fdhila, A.; Dridi, C. Nanoengineering of eco-friendly silver nanoparticles using five different plant extracts and development of cost-effective phenol nanosensor. Sci. Rep. 2021, 11, 22060. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Almohawes, Z.N.; Alahdal, H.; Momenah, M.A.; Bin-Meferij, M.M. Green Synthesis of Hexagonal Silver Nanoparticles Using a Novel Microalgae Coelastrella aeroterrestrica Strain BA_Chlo4 and Resulting Anticancer, Antibacterial, and Antioxidant Activities. Pharmaceutics 2022, 14, 2002. [Google Scholar] [CrossRef]
- Hamida, R.S.; Abdelmeguid, N.E.; Ali, M.A.; Bin-Meferij, M.M.; Khalil, M.I. Synthesis of silver nanoparticles using a novel cyanobacteria Desertifilum sp. extract: Their antibacterial and cytotoxicity effects. Int. J. Nanomed. 2020, 15, 49. [Google Scholar] [CrossRef] [Green Version]
- Boruah, J.S.; Devi, C.; Hazarika, U.; Reddy, P.V.B.; Chowdhury, D.; Barthakur, M.; Kalita, P. Green synthesis of gold nanoparticles using an antiepileptic plant extract: In vitro biological and photo-catalytic activities. RSC Adv. 2021, 11, 28029–28041. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Al-Zaban, M.I. Lethal Mechanisms of Nostoc-Synthesized Silver Nanoparticles Against Different Pathogenic Bacteria. Int. J. Nanomed. 2020, 15, 10499. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef]
- Bin-Meferij, M.M.; Hamida, R.S. Biofabrication and antitumor activity of silver nanoparticles utilizing novel nostoc sp. Bahar M. Int. J. Nanomed. 2019, 14, 9019. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Panda, A.; Rangani, J.; Parida, A.K. Unraveling salt responsive metabolites and metabolic pathways using non-targeted metabolomics approach and elucidation of salt tolerance mechanisms in the xero-halophyte Haloxylon salicornicum. Plant Physiol. Biochem. 2021, 158, 284–296. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Jang, H.-K.; Kim, B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009, 44, 1133–1138. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Nalini, S.K.; Prakash, N.U.; Madhankumar, D. One step green synthesis of silver nano/microparticles using extracts of Trachyspermum ammi and Papaver somniferum. Colloids Surf. B Biointerfaces 2012, 94, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Prathna, T.; Chandrasekaran, N.; Raichur, A.M.; Mukherjee, A. Kinetic evolution studies of silver nanoparticles in a bio-based green synthesis process. Colloids Surf. A Physicochem. Eng. Asp. 2011, 377, 212–216. [Google Scholar] [CrossRef]
- Tripathy, A.; Raichur, A.M.; Chandrasekaran, N.; Prathna, T.; Mukherjee, A. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J. Nanoparticle Res. 2010, 12, 237–246. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, T.; Nadhman, A. Applications of plant terpenoids in the synthesis of colloidal silver nanoparticles. Adv. Colloid Interface Sci. 2016, 234, 132–141. [Google Scholar]
- Thapa, P.; Prakash, O.; Rawat, A.; Kumar, R.; Srivastava, R.; Rawat, D.; Pant, A. Essential Oil Composition, Antioxidant, Anti-inflammatory, Insect Antifeedant and Sprout Suppressant Activity in Essential Oil From Aerial Parts of Cotinus coggygria Scop. J. Essent. Oil Bear. Plants 2020, 23, 65–76. [Google Scholar] [CrossRef]
- Su, Y.-C.; Hsu, K.-P.; Hua, K.-F.; Ho, C.-L. Composition, in vitro anti-inflammatory, antioxidant and antimicrobial activities of essential oils from leaf and twig parts of Cupressus cashmeriana. Nat. Prod. Commun. 2015, 10, 1934578X1501000837. [Google Scholar] [CrossRef]
- Ullah, R.; Alsaid, M.S.; Alqahtani, A.S.; Shahat, A.A.; Naser, A.A.; Mahmood, H.M.; Ahamad, S.R.; Al-Mishari, A.A.; Ahmad, S. Anti-inflammatory, antipyretic, analgesic, and antioxidant activities of Haloxylon salicornicum aqueous fraction. Open Chem. 2019, 17, 1034–1042. [Google Scholar] [CrossRef]
- Dashora, A.; Rathore, K.; Raj, S.; Sharma, K. Synthesis of silver nanoparticles employing Polyalthia longifolia leaf extract and their in vitro antifungal activity against phytopathogen. Biochem. Biophys. Rep. 2022, 31, 101320. [Google Scholar] [CrossRef]
- Lin, Q.; Hong, X.; Zhang, D.; Jin, H. Biosynthesis of size-controlled gold nanoparticles using M. lucida leaf extract and their penetration studies on human skin for plastic surgery applications. J. Photochem. Photobiol. B Biol. 2019, 199, 111591. [Google Scholar] [CrossRef]
- Hung, N.D.; Nam, V.N.; ThiNhan, T.; Dung, T.T.N. Quantitative concentration determination of silver nanoparticles prepared by DC high voltage electrochemical method. Vietnam. J. Chem. 2018, 56, 553–558. [Google Scholar] [CrossRef]
- Dhara, S. Origin of shifts in the surface plasmon resonance frequencies for Au and Ag nanoparticles. Rev. Plasmon. 2015, 2016, 275–294. [Google Scholar]
- Mie, G. A contribution to the optics of turbid media, especially colloidal metallic suspensions. Ann. Phys 1908, 25, 377–445. [Google Scholar] [CrossRef]
- Chelly, M.; Chelly, S.; Zribi, R.; Bouaziz-Ketata, H.; Gdoura, R.; Lavanya, N.; Veerapandi, G.; Sekar, C.; Neri, G. Synthesis of Silver and Gold Nanoparticles from Rumex roseus Plant Extract and Their Application in Electrochemical Sensors. Nanomaterials 2021, 11, 739. [Google Scholar] [CrossRef]
- Arshad, H.; Sadaf, S.; Hassan, U. De-novo fabrication of sunlight irradiated silver nanoparticles and their efficacy against E. coli and S. epidermidis. Sci. Rep. 2022, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Ssekatawa, K.; Byarugaba, D.K.; Kato, C.D.; Wampande, E.M.; Ejobi, F.; Nakavuma, J.L.; Maaza, M.; Sackey, J.; Nxumalo, E.; Kirabira, J.B. Green strategy–based synthesis of silver nanoparticles for antibacterial applications. Front. Nanotechnol. 2021, 59, 697303. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.U.; Rafatullah, M.; Alam, M.; Bogdanchikova, N.; Garibo, D. Search for Effective Approaches to Fight Microorganisms Causing High Losses in Agriculture: Application of P. lilacinum Metabolites and Mycosynthesised Silver Nanoparticles. Biomolecules 2022, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, S.; Astudillo-Calderón, S.; Pintos, B.; Pérez-Urria, E.; Manzanera, J.A.; Martín, L.; Gomez-Garay, A. Role of synthetic plant extracts on the production of silver-derived nanoparticles. Plants 2021, 10, 1671. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.A. Green synthesis of silver nanoparticles using Pseudoduganella eburnea MAHUQ-39 and their antimicrobial mechanisms investigation against drug resistant human pathogens. Int. J. Mol. Sci. 2020, 21, 1510. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, P.; Vikram, K.; Singh, H. Fabrication and characterization of noble crystalline silver nanoparticles from Pimenta dioica leave extract and analysis of chemical constituents for larvicidal applications. Saudi J. Biol. Sci. 2022, 29, 1134–1146. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Mancini, M.C.; Oliveira, F.C.S.d.; Passos, T.M.; Quilty, B.; Thiré, R.M.d.S.M.; McGuinness, G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria 2016, 21, 767–779. [Google Scholar] [CrossRef]
- Nadeem, F.; Fozia, F.; Aslam, M.; Ahmad, I.; Ahmad, S.; Ullah, R.; Almutairi, M.H.; Aleya, L.; Abdel-Daim, M.M. Characterization, Antiplasmodial and Cytotoxic Activities of Green Synthesized Iron Oxide Nanoparticles Using Nephrolepis exaltata Aqueous Extract. Molecules 2022, 27, 4931. [Google Scholar] [CrossRef]
- Nagalingam, M.; Kalpana, V.; Panneerselvam, A. Biosynthesis, characterization, and evaluation of bioactivities of leaf extract-mediated biocompatible gold nanoparticles from Alternanthera bettzickiana. Biotechnol. Rep. 2018, 19, e00268. [Google Scholar]
- Singh, P.; Mijakovic, I. Green synthesis and antibacterial applications of gold and silver nanoparticles from Ligustrum vulgare berries. Sci. Rep. 2022, 12, 7902. [Google Scholar] [CrossRef] [PubMed]
- Elbagory, A.M.; Cupido, C.N.; Meyer, M.; Hussein, A.A. Large scale screening of southern African plant extracts for the green synthesis of gold nanoparticles using microtitre-plate method. Molecules 2016, 21, 1498. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, R.; Vallet-Regí, M. Hard and soft protein corona of nanomaterials: Analysis and relevance. Nanomaterials 2021, 11, 888. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, L.; Guo, C.; Yan, B.; Su, G. Regulating protein corona formation and dynamic protein exchange by controlling nanoparticle hydrophobicity. Front. Bioeng. Biotechnol. 2020, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Ichwan, S.J.; Al-Zikri, P.N.H.; Suriyah, W.H.; Soundharrajan, I.; Govindan, N.; Maniam, G.P.; Yusoff, M.M. In vitro anticancer activity of Au, Ag nanoparticles synthesized using Commelina nudiflora L. aqueous extract against HCT-116 colon cancer cells. Biol. Trace Elem. Res. 2016, 173, 297–305. [Google Scholar] [CrossRef]
- Baran, A.; Fırat Baran, M.; Keskin, C.; Hatipoğlu, A.; Yavuz, Ö.; İrtegün Kandemir, S.; Adican, M.T.; Rovshan, K.; Mammadova, A.; Ahmadian, E. Investigation of antimicrobial and cytotoxic properties and specification of silver nanoparticles (AgNPs) Derived from Cicer arietinum L. green leaf extract. Front. Bioeng. Biotechnol. 2022, 10, 263. [Google Scholar] [CrossRef]
- Salvioni, L.; Galbiati, E.; Collico, V.; Alessio, G.; Avvakumova, S.; Corsi, F.; Tortora, P.; Prosperi, D.; Colombo, M. Negatively charged silver nanoparticles with potent antibacterial activity and reduced toxicity for pharmaceutical preparations. Int. J. Nanomed. 2017, 12, 2517. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnology 2017, 15, 65. [Google Scholar] [CrossRef]
- Okkeh, M.; Bloise, N.; Restivo, E.; De Vita, L.; Pallavicini, P.; Visai, L. Gold nanoparticles: Can they be the next magic bullet for multidrug-resistant bacteria? Nanomaterials 2021, 11, 312. [Google Scholar] [CrossRef]
- Lewis, K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013, 12, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.; Edwards, R. Insights into antibiotic resistance through metagenomic approaches. Future Microbiol. 2012, 7, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, G.; Gobinath, C.; Karpagam, K.; Hemamalini, V.; Premkumar, K.; Sivaramakrishnan, S. Phyto-synthesis of silver nanoscale particles using Morinda citrifolia L. and its inhibitory activity against human pathogens. Colloids Surf. B: Biointerfaces 2012, 95, 235–240. [Google Scholar] [CrossRef]
- Priya Velammal, S.; Devi, T.A.; Amaladhas, T.P. Antioxidant, antimicrobial and cytotoxic activities of silver and gold nanoparticles synthesized using Plumbago zeylanica bark. J. Nanostructure Chem. 2016, 6, 247–260. [Google Scholar] [CrossRef] [Green Version]











| No. | Compound | Retention Time | Area% | Matched Factor | Molecular Formula | Molecular Weight | Chemical Structure |
|---|---|---|---|---|---|---|---|
| 1 | 1-Methyl-2-(1-methylethenyl)-4-(1-methylethylidene)-1-vinylcyclohexane), (1R-trans)- | 10.12 | 0.64 | 846 | C15H24 | 204 |  |
| 2 | Cyclohexane, 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl)-, (1R,2R,4S)-rel- | 11.46 | 1.70 | 880 | C15H24 | 204 |  |
| 3 | Aromandendrene | 12.05, 12.45,12.87, 13.25, 13.45, 13.67, 14.76 | 0.80, 1.38, 0.38, 0.32, 0.70, 1.39, 0.71 | 873, 886, 893, 885, 856, 890, 868 | C15H24 | 204 |  |
| 4 | Beta-longipinene | 13.16 | 0.46 | 828 | C15H24 | 204 |  |
| 5 | Eremophilene | 13.88 | 1.65 | 902 | C15H24 | 204 |  |
| 6 | Alpha-copaene | 14.03 | 1.30 | 788 | C15H24 | 204 |  |
| 7 | Gamma-himachalene | 14.15 | 0.34 | 808 | C15H24 | 204 |  |
| 8 | o-Menth-8-ene-4-methanol, α,α -dimethyl-1-vinyl-, (1S,2S,4R)-(-)- | 14.43, 15.30 | 4.45, 3.18 | 899, 909 | C15H26O | 222 |  |
| 9 | Beta-cadinene | 14.56 | 2.55 | 807 | C15H24 | 204 |  |
| 10 | Beta-guaiene | 14.92, 15.87 | 0.32, 0.31 | 765, 782 | C15H24 | 204 |  |
| 11 | Murolan-3,9(11)-diene-10-peroxy | 15.03 | 0.47 | 760 | C15H24O2 | 236 |  |
| 12 | (-)-Globulol | 15.13 | 0.48 | 829 | C15H26O | 222 |  |
| 13 | Alpha-farnesene | 15.63 | 0.64 | 807 | C15H24 | 204 |  |
| 14 | 10-Epi-gamma-Eudesmol | 16.20, 16.68, 17.10, 17.58 | 6.38, 6.90, 4.17, 6.24 | 870, 898, 865, 898 | C15H26O | 222 |  |
| 15 | Guaiol | 16.46 | 0.31 | 877 | C15H26O | 222 |  |
| 16 | Eudesma-4(14),7(11)-diene | 17.37 | 0.32 | 786 | C15H24 | 204 |  |
| 17 | 1-Chlorooctadecane | 18.49, 20.56 | 1.31, 0.67 | 768, 755 | C18H37Cl | 288 |  |
| 18 | Methyl tetradecanoate | 19.13 | 0.86 | 694 | C15H30O2 | 242 |  |
| 19 | Cadalene | 20.12 | 0.57 | 731 | C15H18 | 198 |  |
| 20 | 2-Acetyl-3-(2-cinnamido)ethyl-7-methoxyindole | 20.68 | 0.52 | 615 | C22H22N2O3 | 362 |  |
| 21 | 1,2-15,16-Diepoxyhexadecane | 21.35 | 0.39 | 717 | C16H30O2 | 254 |  |
| 22 | Phthalic acid, isobutyl octadecyl ester | 22.07 | 0.52 | 828 | C30H50O4 | 474 |  |
| 23 | Ethyl linoleate | 22.18 | 0.45 | 714 | C20H36O2 | 308 |  |
| 24 | 12,15-Octadecadiynoic acid, methyl Ester | 22.72 | 0.49 | 713 | C19H30O2 | 290 |  |
| 25 | Methyl palmitate | 23.22 | 10.50 | 906 | C17H34O2 | 270 |  |
| 26 | n-Hexadecanoic acid | 24.59 | 3.96 | 831 | C16H32O2 | 256 |  |
| 27 | Cyclopropa[d]naphthalen-3-one, octahydro-2,4a,8,8-tetramethyl-, oxime | 25.97, 26.77 | 1.87, 0.57 | 647, 675 | C15H25NO | 235 |  |
| 28 | 8,11-Octadecadienoic acid, methyl Ester | 26.25 | 0.83 | 795 | C19H34O2 | 294 |  |
| 29 | 9-Octadecenoic acid (Z)-, methyl Ester | 26.42, 26.53 | 6.81, 3.62 | 924, 885 | C19H36O2 | 296 |  |
| 30 | Methyl stearate | 26.9 | 5.20 | 865 | C19H38O2 | 298 |  |
| 31 | Methyl 6,8-octadecadiynoate | 27.20 | 0.86 | 730 | C19H30O2 | 290 |  |
| 32 | Oleic Acid | 27.66, 27.99 | 3.04, 1.22 | 812, 774 | C18H34O2 | 282 |  |
| 33 | 1,1-Dichloro-2-(2,2-dichloro-1-methylcyclopropyl)-2-methylcyclopropane | 28.83 | 4.97 | 687 | C8H10Cl4 | 246 |  |
| 34 | Methyl 14-methyl-eicosanoate | 30.25 | 0.65 | 701 | C22H44O2 | 340 |  |
| 35 | Dodecanoic acid, 10-methyl-, methyl Ester | 33.38 | 0.69 | 771 | C14H28O2 | 228 |  |
| 36 | Phthalic acid, 5-methylhex-2-yl heptadecyl ester | 33.67 | 0.93 | 805 | C32H54O4 | 502 |  |
| FTIR Spectra (cm−1) | Functional Gtoups of Haloxylon salicornicum | FTIR Spectra (cm−1) | Functional Gtoups of H@Ag-NPs | FTIR Spectra (cm−1) | Functional Gtoups of H@Au-NPs |
|---|---|---|---|---|---|
| 3368.04 | O-H | 3424.7 | O-H | 3424.7 | O-H |
| 1634.5 | N-H | 2928.8 | C-H | 2928.8 | C-H |
| 1467.1 | C-H | 2861.8 | C-H | 2861.8 | C-H |
| 1409.01 | O-H | 1634.0 | C=C | 1747.4 | C=O |
| 1330.7 | C-N | 1510.3 | N-O | 1634.0 | C=C |
| 1251.4 | C-O | 1386.5 | C-H | 1465.9 | C-H |
| 1093.8 | C-O | 1240.2 | C-N | 1231.0 | C-N |
| 907.4 | C=C | 1059.7 | C-O | 1173.2 | C-O |
| 829.9 | C-Cl | 587.6 | C-Cl | 1059.7 | C-O |
| 721.2 | C=C | 582.0 | C-Cl | ||
| 609.7 | C-Cl | ||||
| 500.0 | C-I |
| H@Ag-NPs | H@Au-NPs | ||||||
|---|---|---|---|---|---|---|---|
| Elements | Line | Mass% | Atom% | Elements | Line | Mass% | Atom% |
| C | K | 2.55 ± 0.01 | 16.44 ± 0.06 | C | K | 3.97 ± 0.02 | 35.88 ± 0.14 |
| O | K | 0.58 ± 0.01 | 2.80 ± 0.06 | Al | K | 0.21 ± 0.01 | 0.85 ± 0.04 |
| Al | K | 0.14 ± 0.01 | 0.41± 0.02 | Cu | K | 5.54 ± 0.08 | 9.45 ± 0.13 |
| Cl | K | 7.08 ± 0.02 | 15.46 ± 0.05 | Zn | K | 3.73 ± 0.09 | 6.18 ± 0.15 |
| Cu | K | 1.04 ± 0.04 | 1.26 ± 0.05 | Au | M | 86.55 ± 0.14 | 47.64 ± 0.08 |
| Ag | L | 88.61 ± 0.10 | 63.62 ± 0.07 | ||||
| Total | 100 | 100 | Total | 100 | 100 | ||
| Bacteria | H@Ag-NPs (µg/mL) | H@Au-NPs (µg/mL) | H. salicornicum (µg/mL) | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| S.aureus | 1.95 | 3.9 | >500 | >500 | >500 | >500 |
| B. cereus | 3.90 | 7.8 | >500 | >500 | >500 | >500 |
| K. pneumoniae | 3.90 | 7.81 | >500 | >500 | >500 | >500 |
| E. coli | 7.8 | 15.6 | >500 | >500 | >500 | >500 |
| Strains | Ciprofloxacin | H@Ag-NPs | H@Au-NPs | H. salicornicum |
|---|---|---|---|---|
| S.aureus | 30.0 ± 0.2 | 17.0 ± 0.1 | 0.0 ± 0.0 | 12.0 ± 0.1 |
| B. cereus | 32.0 ± 0.3 | 13.4 ± 0.2 | 0.0 ± 0.0 | 11.0 ± 0.2 |
| E. coli | 30.0 ± 0.2 | 13.0 ± 0.3 | 0.0 ± 0.0 | 9.8 ± 0.3 |
| K. pneumoniae | 32.0 ± 0.2 | 13.3 ± 0.4 | 0.0 ± 0.0 | 9.9 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamida, R.S.; Ali, M.A.; Alfassam, H.E.; Momenah, M.A.; Alkhateeb, M.A.; Bin-Meferij, M.M. One-Step Phytofabrication Method of Silver and Gold Nanoparticles Using Haloxylon salicornicum for Anticancer, Antimicrobial, and Antioxidant Activities. Pharmaceutics 2023, 15, 529. https://doi.org/10.3390/pharmaceutics15020529
Hamida RS, Ali MA, Alfassam HE, Momenah MA, Alkhateeb MA, Bin-Meferij MM. One-Step Phytofabrication Method of Silver and Gold Nanoparticles Using Haloxylon salicornicum for Anticancer, Antimicrobial, and Antioxidant Activities. Pharmaceutics. 2023; 15(2):529. https://doi.org/10.3390/pharmaceutics15020529
Chicago/Turabian StyleHamida, Reham Samir, Mohamed Abdelaal Ali, Haifa Essa Alfassam, Maha Abdullah Momenah, Mariam Abdulaziz Alkhateeb, and Mashael Mohammed Bin-Meferij. 2023. "One-Step Phytofabrication Method of Silver and Gold Nanoparticles Using Haloxylon salicornicum for Anticancer, Antimicrobial, and Antioxidant Activities" Pharmaceutics 15, no. 2: 529. https://doi.org/10.3390/pharmaceutics15020529
APA StyleHamida, R. S., Ali, M. A., Alfassam, H. E., Momenah, M. A., Alkhateeb, M. A., & Bin-Meferij, M. M. (2023). One-Step Phytofabrication Method of Silver and Gold Nanoparticles Using Haloxylon salicornicum for Anticancer, Antimicrobial, and Antioxidant Activities. Pharmaceutics, 15(2), 529. https://doi.org/10.3390/pharmaceutics15020529