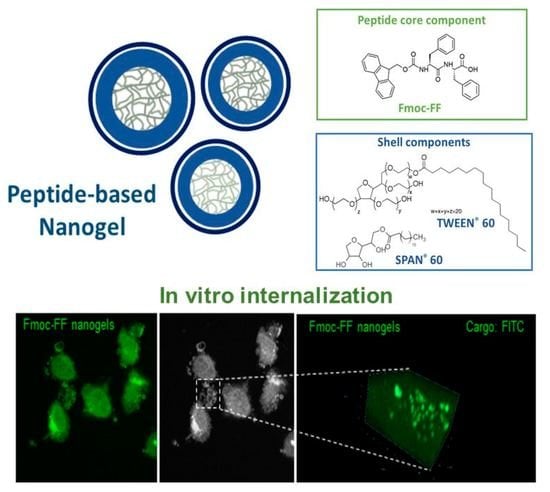
Caveolin-Mediated Internalization of Fmoc-FF Nanogels in Breast Cancer Cell Lines
Abstract
:1. Introduction
2. Experimental Section
2.1. Formulation of Fmoc-FF Nanogels and FITC Loaded Fmoc-FF Nanogels
2.2. Dynamic Light Scattering (DLS) Measurements
2.3. Nanoparticles Tracking Analysis (NTA) Measurement
2.4. Cell Culture
2.5. RNA Extraction and RT-PCR Analysis
- RPS18:fw 5′-CGATGGGCGGCGGAAAATA-3′; rev 5-CTGCTTTCCTCAACACCACA-3′
- CyclinA:fw 5′-AAATGGGCAGTACAGGAGGA-3′; rev 5′-CCACAGTCAGGGAGTGCTTT-3′
- CyclinB:fw 5′-CATGGTGCACTTTCCTCCTT-3′; rev 5′ AGGTAATGTTGTAGAGTTGGTGTCC-3′
- CyclinD:fw 5′-GCTGTGCATCTACACCGACA-3′; rev 5′-TTGAGCTTGTTCACCAGGAG-3′
- CyclinE:fw 5′-GGCCAAAATCGACAGGAC-3′; rev 5′-GGGTCTGCACAGACTGCAT-3′
- Caveolin 1:fw 5’-ACACGGCTGATGCACTGAACTC-3’; rev 5’-GACACACAGGGAAGACCAAGACG-3’
2.6. Cell Cycle Analysis
2.7. MTT Assays
2.8. Immunofluorescence Analysis of Fmoc-FF Nanogels Uptake
2.9. Statistical Analyses
3. Results
3.1. Physicochemical Details of Fmoc-FF Nanogels
3.2. Fmoc-FF Nanogels Promotes Growth Arrest of MDA-MB-231 Cells
3.3. Fmoc-FF Nanogel Enters MDA-MB-231 Cells via Caveolae-Mediated Endocytosis
3.4. Human Serum Albumin (HSA) Overload Saturates the Caveolae and Blocks the Fmoc-FF Nanogels Entering in MDA-MB-231
3.5. The Fmoc-FF Nanogels Selectivity toward MDA-MB-231 Results from Caveolin-1 Overexpression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, P.; Chen, G.; Zhang, J. A review of liposomes as a drug delivery system: Current status of approved products, regulatory environments, and future perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.; Diaferia, C.; Balasco, N.; Sibillano, T.; Roviello, V.; Giannini, C.; Vitagliano, L.; Morelli, G.; Accardo, A. Fabrication of fluorescent nanospheres by heating PEGylated tetratyrosine nanofibers. Sci. Rep. 2021, 11, 2470. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Pan, S.; Rodrigues, J.; Elkodous, M.A.; Danquahg, M.K. Medical applications of biopolymer nanofibers. Biomater. Sci. 2022, 10, 4107–4118. [Google Scholar] [CrossRef]
- Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D. Polymer-based nanofiber–nanoparticle hybrids and their medical applications. Polymers 2022, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Tesauro, D.; Aloj, L.; Tarallo, L.; Arra, C.; Mangiapia, G.; Vaccaro, M.; Pedone, C.; Paduano, L.; Morelli, G. Peptide-containing aggregates as selective nanocarriers for therapeutics. ChemMedChem 2008, 3, 594–602. [Google Scholar] [CrossRef]
- Accardo, A.; Mansi, R.; Salzano, G.; Morisco, A.; Aurilio, M.; Parisi, A.; Maione, F.; Cicala, C.; Ziaco, B.; Tesauro, D.; et al. Bombesin peptide antagonist for target-selective delivery of liposomal doxorubicin on cancer cells. J. Drug Target. 2013, 21, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Salmaso, S.; Caliceti, P. Stealth properties to improve therapeutic efficacy of drug nanocarriers. J. Drug Deliv. 2013, 2013, 374252. [Google Scholar] [CrossRef]
- Ravi Kiran, A.V.V.V.; Kusuma Kumari, G.; Krishnamurthy, P.T.; Khaydarov, R.R. Tumor microenvironment and nanotherapeutics: Intruding the tumor fort. Biomater. Sci. 2021, 9, 7667–7704. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef]
- Gallo, E.; Rosa, E.; Diaferia, C.; Rossi, F.; Tesauro, D.; Accardo, A. Systematic overview of soft materials as a novel frontier for MRI contrast agents. RSC Adv. 2020, 10, 27064–27080. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Gul, M.; Nguyen, T.T.L.; Maeng, H.J. Controlled release and targeted drug delivery with poly (lactic-co-glycolic acid) nanoparticles: Reviewing two decades of research. J. Pharm. Investig. 2022, 52, 683–724. [Google Scholar] [CrossRef]
- Mukai, H.; Ogawa, K.; Kato, N.; Kawakami, S. Recent advances in lipid nanoparticles for delivery of nucleic acid, mRNA, and gene editing-based therapeutics. Drug Metabol. Pharmacokinet. 2022, 44, 100450. [Google Scholar] [CrossRef] [PubMed]
- Yee Kuen, C.; Masarudin, M.J. Chitosan nanoparticle-based system: A new insight into the promising controlled release system for lung cancer treatment. Molecules 2022, 27, 473. [Google Scholar] [CrossRef] [PubMed]
- Sivaram, A.J.; Rajitha, P.; Maya, S.; Jayakumar, R.; Sabitha, M. Nanogels for delivery, imaging and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 509–533. [Google Scholar] [CrossRef]
- Nik, M.E.; Malaekeh-Nikouei, B.; Amin, M.; Hatamipour, M.; Teymouri, M.; Sadeghnia, H.Z.; Iranshahi, M.; Jaafari, M.Z. Liposomal formulation of galbanic acid improved therapeutic efficacy of PEGylated liposomal doxorubicin in mouse colon carcinoma. Sci. Rep. 2019, 9, 9527. [Google Scholar] [CrossRef] [Green Version]
- Haghiralsadat, F.; Amoabediny, G.; Sheikhha, M.H.; Zandieh-doulabi, B.; Naderinezhad, S.; Helder, M.N.; Forouzanfar, T. New liposomal doxorubicin nanoformulation for osteosarcoma: Drug release kinetic study based on thermo and pH sensitivity. Chem. Biol. Drug Des. 2017, 90, 368–379. [Google Scholar] [CrossRef]
- Joniec, A.; Sek, S.; Krysinski, P. Magnetoliposomes as potential carriers of doxorubicin to tumours. Chem. Eur. J. 2016, 22, 17715–17724. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Carniato, F.; Tei, L.; Botta, M.; Ravera, E.; Fragai, M.; Parigi, G.; Luchinat, C. 1H NMR Relaxometric Study of Chitosan-Based Nanogels Containing Mono- and Bis-Hydrated Gd(III) Chelates: Clues for MRI Probes of Improved Sensitivity. ACS Appl. Bio Mater. 2020, 3, 9065–9072. [Google Scholar] [CrossRef]
- Lu, D.-Q.; Liu, D.; Liu, J.; Li, W.X.; Ai, Y.; Wang, J.; Guan, D. Facile synthesis of chitosan-based nanogels through photo-crosslinking for doxorubicin delivery. Int. J. Biol. Macromol. 2022, 218, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Atallah, M.A.; Sallam, M.A.; Abdelmoneem, M.A.; Teleb, M.; Elkhodairy, K.A.; Bekhit, A.A.; Khafaga, A.F.; Noreldin, A.E.; Elzoghby, A.O.; Khattab, S.N. Green self-assembled lactoferrin carboxymethyl cellulose nanogels for synergistic chemo/herbal breast cancer therapy. Colloids Surf. B Biointerfaces 2022, 217, 112657. [Google Scholar] [CrossRef]
- Simakova, A.; Averick, S.; Jazani, A.M.; Matyjaszewski, K. Controlling size and surface chemistry of cationic nanogels by inverse microemulsion ATRP. Macromol. Chem. Phys. 2022, 224, 2200210. [Google Scholar] [CrossRef]
- Gao, D.; Asghar, S.; Ye, J.; Zhang, M.; Hu, R.; Wang, Y.; Huang, L.; Yuan, C.; Chen, Z.; Xiao, Y. Dual-targeted enzyme-sensitive hyaluronic acid nanogels loading paclitaxel for the therapy of breast cancer. Carbohydr. Polym. 2022, 294, 119785. [Google Scholar] [CrossRef]
- Xu, F.; Xu, B.; Chen, H.; Ju, X.; De Mejia, E.G. Enhancement of DPP-IV inhibitory activity and the capacity for enabling GLP-1 secretion through RADA16-assisted molecular designed rapeseed peptide nanogels. Food Funct. 2022, 13, 5215–5228. [Google Scholar] [CrossRef]
- Rosa, E.; Diaferia, C.; Gallo, E.; Morelli, G.; Accardo, A. Stable formulations of peptide-based nanogels. Molecules 2020, 25, 3455. [Google Scholar] [CrossRef]
- Diaferia, C.; Rosa, E.; Morelli, G.; Accardo, A. Fmoc-diphenylalanine hydrogels: Optimization of preparation methods and structural insights. Pharmaceuticals 2022, 15, 1048. [Google Scholar] [CrossRef]
- Gallo, E.; Diaferia, C.; Rosa, E.; Smaldone, G.; Morelli, G.; Accardo, A. Peptide-based hydrogels and nanogels for delivery of doxorubicin. Int. J. Nanomed. 2021, 16, 1617–1630. [Google Scholar] [CrossRef]
- Bachurski, D.; Schuldner, M.; Nguyen, P.-H.; Malz, A.; Reiners, K.S.; Grenzi, P.C.; Babatz, F.; Schauss, A.C.; Hansen, H.P.; Hallek, M.; et al. Extracellular vesicle measurements with nanoparticle tracking analysis—An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. J. Extracell. Vesicles 2019, 8, 1596016. [Google Scholar] [CrossRef] [PubMed]
- Coppola, L.; Baselice, S.; Messina, F.; Giannatiempo, R.; Farina, A.; Vitagliano, L.; Smaldone, G.; Salvatore, M. KCTD15 Is Overexpressed in her2+ Positive Breast Cancer Patients and Its Silencing Attenuates Proliferation in SKBR3 cell line. Diagnostics 2022, 12, 591. [Google Scholar] [CrossRef]
- Riccio, G.; Bottone, S.; La Regina, G.; Badolati, N.; Passacantilli, S.; Rossi, S.B.; Accardo, A.; Dentice, M.; Silvestri, R.; Novellino, E.; et al. A negative allosteric modulator of WNT receptor frizzled 4 switches into an Allosteric Agonist. Biochemistry 2018, 57, 839–851. [Google Scholar] [CrossRef]
- Lemma, V.; D’Agostino, M.; Caporaso, M.; Mallardo, M.; Oliviero, G.; Stornaiuolo, M.; Bonatti, S. A disorder-to-order structural transition in the COOH-tail of Fz4 determines misfolding of the L501fsX533-Fz4 mutant. Sci. Rep. 2013, 3, 2659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radner, S.; Celie, P.H.N.; Fuchs, K.; Sieghart, W.; Sixma, T.K.; Stornaiuolo, M. Transient transfection coupled to baculovirus infection for rapid protein expression screening in insect cells. J. Struct. Biol. 2012, 179, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Càceres, J.; Caballero-Díaz, D.; Nwosu, Z.C.; Meyer, C.; López-Luque, J.; Malfettone, A.; Lastra, R.; Serrano, T.; Ramos, E.; Dooley, S.; et al. The level of caveolin-1 expression determines response to TGF-β as a tumour suppressor in hepatocellular carcinoma cells. Cell Death Dis. 2017, 8, e3098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.-W.; Zu, X.-Y.; Tuo, Q.-H.; Chen, L.-X.; Lei, X.-Y.; Li, K.; Tang, C.-K.; Liao, D.-F. Caveolae and caveolin-1 mediate endocytosis and transcytosis of oxidized low density lipoprotein in endothelial cells. Acta Pharmacol. Sin. 2010, 31, 1336–1342. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Jin, M.-R.; Wang, S.-J.; Xie, X.-T.; Hu, W.; Tang, H.-F.; Liu, B. Co-delivery of paclitaxel and doxorubicin using polypeptide-engineered nanogels for combination therapy of tumor. Nanotechnology 2022, 33, 155101. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yue, T.; Wang, C.; Fan, Z.; Gazit, E.; Du, J. Ultrasound-responsive peptide nanogels to balance conflicting requirements for deep tumor penetration and prolonged blood circulation. ACS Nano 2022, 16, 9183–9194. [Google Scholar] [CrossRef]
- Arab, W.T.; Susapto, H.H.; Alhattab, D.; Hauser, C.A.E. Peptide nanogels as a scaffold for fabricating dermal grafts and 3D vascularized skin models. J. Tissue Eng. 2022, 13, 20417314221111868. [Google Scholar] [CrossRef]
- Sousa de Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Azadmanesh, K.; Shokrgozar, M.A.; Journeay, W.S.; Laurent, S. Effect of nanoparticles on the cell life cycle. Chem. Rev. 2011, 111, 3407–3432. [Google Scholar] [CrossRef] [PubMed]
- Billiet, L.; Gomez, J.P.; Berchel, M.; Jaffres, P.A.; Le, G.T.; Montier, T.; Bertrand, E.; Cheradame, H.; Guegan, P.; Mevel, M.; et al. Gene transfer by chemical vectors, and endocytosis routes of polyplexes, lipoplexes and lipopolyplexes in a myoblast cell line. Biomaterials 2012, 33, 2980–2990. [Google Scholar] [CrossRef]
- Sahay, G.; Kim, J.O.; Kabanov, A.V.; Bronich, T.K. The exploitation of differential endocytic pathways in normal and tumor cells in the selective targeting of nanoparticulate chemotherapeutic agents. Biomaterials 2010, 31, 923–933. [Google Scholar] [CrossRef] [Green Version]
- Bohmer, N.; Jordan, A. Caveolin-1 and CDC42 mediated endocytosis of silica-coated iron oxide nanoparticles in HeLa cells. J. Nanotechnol. 2015, 6, 167–176. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, Y.; Li, W.; Kang, W.; Tang, R.; Wu, W.; Xing, Z.; Zhou, L. The function of Cav-1 in MDA-MB-231 breast cancer cell migration and invasion induced by ectopic ATP5B. Med. Oncol. 2021, 38, 73. [Google Scholar] [CrossRef] [PubMed]
- Cano-Cortes, M.V.; Altea-Manzano, P.; Laz-Ruiz, J.A.; Unciti-Broceta, J.-D.; Lopez-Delgado, F.J.; Espejo-Roman, J.M.; Diaz-Mochon, J.J.; Sanchez-Martin, R.M. An effective polymeric nanocarrier that allows for active targeting and selective drug delivery in cell coculture systems. Nanoscale 2021, 13, 3500–3511. [Google Scholar] [CrossRef]
- Prasad Thelu, H.V.; Atchimnaidu, S.; Perumal, D.; Harikrishnan, K.S.; Vijayan, S. Self-assembly of an aptamer-decorated, DNA–protein hybrid nanogel: A biocompatible nanocarrier for targeted cancer therapy. ACS Appl. Bio Mater. 2019, 2, 5227–5234. [Google Scholar] [CrossRef]
- Solin, K.; Beaumont, M.; Rosenfeldt, S.; Orelma, H.; Borghei, M.; Bacher, M.; Opietnik, M.; Rojas, O.J. Self-assembly of soft cellulose nanospheres into colloidal gel layers with enhanced protein adsorption capability for next-generation immunoassays. Small 2020, 16, 2004702. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, H.; Rao, Z.; Li, H.; Wu, Y.; Zhao, J.; Rong, J. Controlled protein adsorption and delivery of thermosensitive poly(N-isopropylacrylamide) nanogels. J. Mater. Chem. B 2017, 5, 7974–7984. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smaldone, G.; Rosa, E.; Gallo, E.; Diaferia, C.; Morelli, G.; Stornaiuolo, M.; Accardo, A. Caveolin-Mediated Internalization of Fmoc-FF Nanogels in Breast Cancer Cell Lines. Pharmaceutics 2023, 15, 1026. https://doi.org/10.3390/pharmaceutics15031026
Smaldone G, Rosa E, Gallo E, Diaferia C, Morelli G, Stornaiuolo M, Accardo A. Caveolin-Mediated Internalization of Fmoc-FF Nanogels in Breast Cancer Cell Lines. Pharmaceutics. 2023; 15(3):1026. https://doi.org/10.3390/pharmaceutics15031026
Chicago/Turabian StyleSmaldone, Giovanni, Elisabetta Rosa, Enrico Gallo, Carlo Diaferia, Giancarlo Morelli, Mariano Stornaiuolo, and Antonella Accardo. 2023. "Caveolin-Mediated Internalization of Fmoc-FF Nanogels in Breast Cancer Cell Lines" Pharmaceutics 15, no. 3: 1026. https://doi.org/10.3390/pharmaceutics15031026
APA StyleSmaldone, G., Rosa, E., Gallo, E., Diaferia, C., Morelli, G., Stornaiuolo, M., & Accardo, A. (2023). Caveolin-Mediated Internalization of Fmoc-FF Nanogels in Breast Cancer Cell Lines. Pharmaceutics, 15(3), 1026. https://doi.org/10.3390/pharmaceutics15031026