Nanocarriers for the Delivery of Neuroprotective Agents in the Treatment of Ocular Neurodegenerative Diseases
Abstract
:1. Introduction
2. Methodology
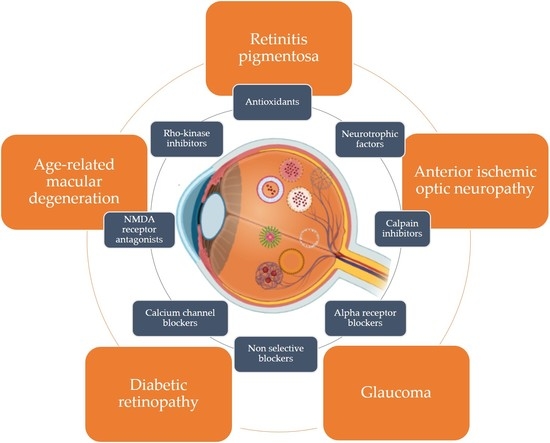
3. Neurodegenerative Diseases Affecting the Eye
3.1. Degenerative Disease of the Photoreceptors
3.2. Diabetic Retinopathy (DR)
3.3. Retinal Ganglion Cell Disease
4. Neuroprotective Agents
5. Barriers to Ocular Drug Delivery

| Drug | M.W (g/mol) | H Bond Donor Value | H Bond Acceptor Value | Log P | TPSA A˚2 | P-Glycoprotein Substrate | Log s | Rule of 5 | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Memantine | 179.3 | 1 | 1 | 2.07 | 26 | Substrate | −3.6 | Yes | [135] |
| Dizocilpine | 221.30 | 1 | 1 | 3.14 | 12 | Substrate | - | Yes | [136] |
| Riluzole | 234.20 | 1 | 7 | 3.43 | 76.4 | Substrate | −3.8 | Yes | [137] |
| Brimonidine | 292.12 | 2 | 3 | 1.7 | 62.2 | Substrate | −3.3 | Yes | [138] |
| Co enzyme Q10 | 863.3 | 0 | 4 | 10 | 52.6 | Substrate | −3 | Yes | [139] |
| Citicoline | 488.32 | 3 | 11 | −1.4 | 214 | Substrate | −1.8 | Yes | [140] |
| Flunarizine | 404.5 | 2 | 3 | 5.75 | 6.5 | Substrate | −5.4 | No | [141] |
| Lomerizine | 468.5 | 0 | 7 | 5.08 | 34.2 | Inhibitor | −4.8 | No | [142] |
| Minocycline | 457.5 | 5 | 9 | 0.05 | 165 | Substrate | −2.2 | Yes | [143] |
| Lutein | 568.9 | 2 | 2 | 7.9 | 40.5 | Substrate | −5.9 | No | [144] |
| Ginkgo biloba extract | 358.6 | 1 | 1 | 1.06 | 20.2 | Inhibitor | - | - | [145] |
| Vitamin C | 176.12 | 4 | 6 | −1.85 | 107 | Non-Substrate | 0.14 | Yes | [146] |
| Vitamin E | 430.7 | 1 | 2 | 12.2 | 29.5 | Substrate | −7.6 | No | [74] |
| Palmitoyl-ethanolamide | 299.5 | 2 | 2 | - | 49.3 | - | - | - | [147] |
| Melatonin | 232.28 | 2 | 2 | 1.6 | 54.1 | Substrate | −3.2 | Yes | [148] |
| Taurine | 125.15 | 2 | 4 | −3.36 | 88.8 | Non-substrate | −0.08 | Yes | [149] |
| Resveratrol | 228.24 | 3 | 3 | 3.10 | 60.7 | Non-substrate | −3.5 | Yes | [150] |
| Forskolin | 410.5 | 3 | 7 | 1.36 | 113 | Substrate | −2.6 | Yes | [151] |
| Curcumin | 368.4 | 2 | 6 | 3.29 | 93.1 | Substrate | −4.8 | Yes | [152] |
| Saffron | 977 | 14 | 24 | - | 391 | [153] | |||
| Timolol maleate | 432.5 | 4 | 12 | 183 | Substrate | −3.1 | Yes | [154] | |
| Azithromycin | 749 | 5 | 14 | 4.02 | 180 | Substrate | −3.2 | No | [155] |
| Ampicillin | 394.4 | 3 | 6 | 1.35 | 138 | Substrate | −2.8 | Yes | [156] |
| Ofloxacin | 361.4 | 1 | 8 | −0.39 | 73.3 | Substrate | −2.4 | Yes | [157] |
| Fluorometholone | 376.5 | 2 | 5 | 2 | 74.6 | Substrate | −4.4 | Yes | [158] |
| Voriconazole | 349.31 | 1 | 8 | 1 | 76.7 | Substrate | −3.6 | Yes | [159] |
| Triamcinolone acetonide | 462.5 | 1 | 8 | 2.31 | 99.1 | Substrate | −2.7 | Yes | [160] |
| Natamycin | 665.7 | 7 | 14 | 1.1 | 231 | Substrate | −3.4 | No | [161] |
| Spironolactone | 416.6 | 0 | 5 | 2.78 | 85.7 | Substrate | −5.3 | Yes | [162] |
| Fluconazole | 306.27 | 1 | 7 | 0.5 | 81.6 | Non-substrate | −2.3 | Yes | [163] |
| Moxifloxacin | 401.4 | 2 | 8 | 2.9 | 82.1 | Substrate | −3.4 | Yes | [164] |
| Propranolol hydrochloride | 295.80 | 3 | 6 | −0.45 | 41.5 | Substrate | −3.5 | Yes | [165] |
| Cysteamine | 77.15 | 2 | 2 | 0.1 | 27 | Substrate | −2.4 | No | [166] |
| Cyclosporin A | 1202.6 | 5 | 12 | 1.4 | 279 | substrate | −0.5 | Yes | [167] |
| Dexamethasone | 392.5 | 3 | 6 | 1.83 | 94.8 | Substrate | −3.9 | Yes | [168] |
| Ketorolac tromethamine | 376.4 | 5 | 7 | 2.28 | 146 | Non-substrate | −2.7 | Yes | [169] |
6. Nanocarrier Systems in the Delivery of Neuroprotective Agents
6.1. Lipid Nanoparticles
6.2. Liposomes
6.3. Polymeric Nanoparticles
6.4. Nanoemulsions and Microemulsions
6.5. Polymeric Nanomicelles
6.6. Dendrimers
7. Limitations of Nanocarriers for Ocular Applications
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crair, M.C.; Mason, C.A. Reconnecting Eye to Brain. J. Neurosci. 2016, 36, 10707–10722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory, R.L. Eye and Brain: The Psychology of Seeing; Princeton University Press: Princeton, NJ, USA, 2015; Volume 80, ISBN 1400866863. [Google Scholar]
- Schnitzer, M.J.; Meister, M. Multineuronal Firing Patterns in the Signal from Eye to Brain. Neuron 2003, 37, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, K.-G.; Bergert, H.; Funk, R.H.W. Neurodegenerative Diseases of the Retina and Potential for Protection and Recovery. Curr. Neuropharmacol. 2008, 6, 164–178. [Google Scholar] [CrossRef] [Green Version]
- Sparrow, J.R.; Zhou, J.; Ben-Shabat, S.; Vollmer, H.; Itagaki, Y.; Nakanishi, K. Involvement of Oxidative Mechanisms in Blue-Light-Induced Damage to A2E-Laden RPE. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1222–1227. [Google Scholar]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neufeld, A.H.; Liu, B. Glaucomatous Optic Neuropathy: When Glia Misbehave. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2003, 9, 485–495. [Google Scholar] [CrossRef]
- Greenwood, J. Experimental Manipulation of the Blood-Brain” and Blood-Retinal Barriers. In Physiology and Pharmacology of the Blood-Brain Barrier; Springer: Berlin/Heidelberg, Germany, 1992; pp. 459–486. [Google Scholar]
- Bosma, E.K.; van Noorden, C.J.F.; Schlingemann, R.O.; Klaassen, I. The Role of Plasmalemma Vesicle-Associated Protein in Pathological Breakdown of Blood–Brain and Blood–Retinal Barriers: Potential Novel Therapeutic Target for Cerebral Edema and Diabetic Macular Edema. Fluids Barriers CNS 2018, 15, 24. [Google Scholar] [CrossRef] [Green Version]
- Chan-Ling, T.; Hughes, S.; Baxter, L.; Rosinova, E.; McGregor, I.; Morcos, Y.; Van Nieuwenhuyzen, P.; Hu, P. Inflammation and Breakdown of the Blood–Retinal Barrier during “Physiological Aging” in the Rat Retina: A Model for CNS Aging. Microcirculation 2007, 14, 63–76. [Google Scholar] [CrossRef]
- Tisi, A.; Feligioni, M.; Passacantando, M.; Ciancaglini, M.; Maccarone, R. The Impact of Oxidative Stress on Blood-Retinal Barrier Physiology in Age-Related Macular Degeneration. Cells 2021, 10, 64. [Google Scholar] [CrossRef]
- Kollias, A.N.; Ulbig, M.W. Diabetic Retinopathy: Early Diagnosis and Effective Treatment. Dtsch. Arztebl. Int. 2010, 107, 75. [Google Scholar]
- Marchesi, N.; Fahmideh, F.; Boschi, F.; Pascale, A.; Barbieri, A. Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas. Cells 2021, 10, 2394. [Google Scholar] [CrossRef] [PubMed]
- Alqawlaq, S.; Huzil, J.T.; Ivanova, M.V.; Foldvari, M. Challenges in Neuroprotective Nanomedicine Development: Progress towards Noninvasive Gene Therapy of Glaucoma. Nanomedicine 2012, 7, 1067–1083. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Dilnawaz, F.; Krishnakumar, S. Nanotechnology in Ocular Drug Delivery. Drug Discov. Today 2008, 13, 144–151. [Google Scholar] [CrossRef]
- Lv, X.; Xia, Y.; Finel, M.; Wu, J.; Ge, G.; Yang, L. Recent Progress and Challenges in Screening and Characterization of UGT1A1 Inhibitors. Acta Pharm. Sin. B 2019, 9, 258–278. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; binti Abd Aziz, A.; Bhattamisra, S.K.; Gorain, B.; Su, J.S.T.; Tan, C.L.; Chin, W.Y.; Yip, K.Y. Potential of Stimuli-Responsive in Situ Gel System for Sustained Ocular Drug Delivery: Recent Progress and Contemporary Research. Polymers 2021, 13, 1340. [Google Scholar] [CrossRef] [PubMed]
- Laddha, U.D.; Mahajan, H.S.; Patel, R.C. An Insight to Ocular in Situ Gelling Systems. Int. J. Adv. Pharm. 2017, 6, 31–40. [Google Scholar]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent Advances in Ocular Drug Delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef] [PubMed]
- Fulton, A.B.; Hansen, R.M.; Moskowitz, A.; Akula, J.D. The Neurovascular Retina in Retinopathy of Prematurity. Prog. Retin. Eye Res. 2009, 28, 452–482. [Google Scholar] [CrossRef] [Green Version]
- Keltner, J.L.; Roth, A.M.; Chang, R.S. Photoreceptor Degeneration: Possible Autoimmune Disorder. Arch. Ophthalmol. 1983, 101, 564–569. [Google Scholar] [CrossRef]
- Strauss, O. The Retinal Pigment Epithelium. Webvision: The Organization of the Retina and Visual System [Internet]. 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK54392/ (accessed on 25 November 2022).
- Kittredge, A.; Zhang, Y.; Yang, T. Evaluating BEST1 Mutations in Pluripotent Stem Cell-Derived Retinal Pigment Epithelial Cells. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 654, pp. 365–382. ISBN 0076-6879. [Google Scholar]
- Sabel, B.A.; Flammer, J.; Merabet, L.B. Residual Vision Activation and the Brain-Eye-Vascular Triad: Dysregulation, Plasticity and Restoration in Low Vision and Blindness–a Review. Restor. Neurol. Neurosci. 2018, 36, 767–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Aung, M.H.; Prunty, M.C.; Hanif, A.M.; Hutson, L.M.; Boatright, J.H.; Pardue, M.T. Tauroursodeoxycholic Acid Protects Retinal and Visual Function in a Mouse Model of Type 1 Diabetes. Pharmaceutics 2021, 13, 1154. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.F.; Joo, K.; Kemp, J.A.; Fialho, S.L.; da Silva Cunha Jr, A.; Woo, S.J.; Kwon, Y.J. Molecular Genetics and Emerging Therapies for Retinitis Pigmentosa: Basic Research and Clinical Perspectives. Prog. Retin. Eye Res. 2018, 63, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Morell, F.; Villagrasa, V.; Ortega, T.; Acero, N.; Muñoz-Mingarro, D.; González-Rosende, M.E.; Castillo, E.; Sanahuja, M.A.; Soriano, P.; Martínez-Solís, I. Medicinal Plants and Natural Products as Neuroprotective Agents in Age-Related Macular Degeneration. Neural Regen. Res. 2020, 15, 2207. [Google Scholar]
- Srilekha, S. Genetic Analysis of Consanguineous South Indian Families with Leber Congenital Amaurosis and Retinitis Pigmentosa Using Homozygosity Mapping. Ph.D. Dissertation, BITS Pilani, Pilani, India, 2016. [Google Scholar]
- Xu, J.; Peng, Q. Retinitis Pigmentosa Treatment with Western Medicine and Traditional Chinese Medicine Therapies. J. Ophthalmol. 2015, 2015, 421269. [Google Scholar] [CrossRef]
- Zhang, X.; Sivaprasad, S. Drusen and Pachydrusen: The Definition, Pathogenesis, and Clinical Significance. Eye 2021, 35, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Monika, F.; DL, K.T.; Guymer, R.H.; Usha, C.; Steffen, S.-V.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-Related Macular Degeneration (Primer). Nat. Rev. Dis. Prim. 2021, 7, 31. [Google Scholar]
- Bhutto, I.; Lutty, G. Understanding Age-Related Macular Degeneration (AMD): Relationships between the Photoreceptor/Retinal Pigment Epithelium/Bruch’s Membrane/Choriocapillaris Complex. Mol. Aspects Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, R.A.; Mousavi, M. Overview of Risk Factors for Age-Related Macular Degeneration (AMD). J. Stem Cells 2015, 10, 171. [Google Scholar]
- Inana, G.; Murat, C.; An, W.; Yao, X.; Harris, I.R.; Cao, J. RPE Phagocytic Function Declines in Age-Related Macular Degeneration and Is Rescued by Human Umbilical Tissue Derived Cells. J. Transl. Med. 2018, 16, 63. [Google Scholar] [CrossRef] [Green Version]
- Van Zyl, T.; Yan, W.; McAdams, A.; Peng, Y.-R.; Shekhar, K.; Regev, A.; Juric, D.; Sanes, J.R. Cell Atlas of Aqueous Humor Outflow Pathways in Eyes of Humans and Four Model Species Provides Insight into Glaucoma Pathogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 10339–10349. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation with Vitamins C and E and Beta Carotene for Age-Related Cataract and Vision Loss: AREDS Report No. 9. Arch. Ophthalmol. 2001, 119, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Ferris III, F.L.; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Committee, B.I. for M.R.C. Clinical Classification of Age-Related Macular Degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Amoaku, W.M.; Chakravarthy, U.; Gale, R.; Gavin, M.; Ghanchi, F.; Gibson, J.; Harding, S.; Johnston, R.L.; Kelly, S.; Lotery, A. Defining Response to Anti-VEGF Therapies in Neovascular AMD. Eye 2015, 29, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Reiter, C.E.N.; Wu, X.; Sandirasegarane, L.; Nakamura, M.; Gilbert, K.A.; Singh, R.S.J.; Fort, P.E.; Antonetti, D.A.; Gardner, T.W. Diabetes Reduces Basal Retinal Insulin Receptor Signaling: Reversal with Systemic and Local Insulin. Diabetes 2006, 55, 1148–1156. [Google Scholar] [CrossRef]
- Pelaez-Luna, M.; Takahashi, N.; Fletcher, J.G.; Chari, S.T. Resectability of Presymptomatic Pancreatic Cancer and Its Relationship to Onset of Diabetes: A Retrospective Review of CT Scans and Fasting Glucose Values Prior to Diagnosis. Off. J. Am. Coll. Gastroenterol. ACG 2007, 102, 2157–2163. [Google Scholar] [CrossRef]
- Do, D.V.; Wang, X.; Vedula, S.S.; Marrone, M.; Sleilati, G.; Hawkins, B.S.; Frank, R.N. Blood Pressure Control for Diabetic Retinopathy. Cochrane Database Syst. Rev. 2015, 1, CD006127. [Google Scholar]
- Garhöfer, G.; Zawinka, C.; Resch, H.; Kothy, P.; Schmetterer, L.; Dorner, G.T. Reduced Response of Retinal Vessel Diameters to Flicker Stimulation in Patients with Diabetes. Br. J. Ophthalmol. 2004, 88, 887–891. [Google Scholar] [CrossRef] [Green Version]
- Little, K.; Llorián-Salvador, M.; Scullion, S.; Hernández, C.; Simó-Servat, O.; Del Marco, A.; Bosma, E.; Vargas-Soria, M.; Carranza-Naval, M.J.; Van Bergen, T. Common Pathways in Dementia and Diabetic Retinopathy: Understanding the Mechanisms of Diabetes-Related Cognitive Decline. Trends Endocrinol. Metab. 2022, 33, 50–71. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kowluru, A.; Mishra, M.; Kumar, B. Oxidative Stress and Epigenetic Modifications in the Pathogenesis of Diabetic Retinopathy. Prog. Retin. Eye Res. 2015, 48, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.N.; Schulz, L.; Abe, K.; Iezzi, R. Temporal Variation in Diabetic Macular Edema Measured by Optical Coherence Tomography. Ophthalmology 2004, 111, 211–217. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Cheung, C.Y.-L.; Lim, G.; Tan, G.S.W.; Quang, N.D.; Gan, A.; Hamzah, H.; Garcia-Franco, R.; San Yeo, I.Y.; Lee, S.Y. Development and Validation of a Deep Learning System for Diabetic Retinopathy and Related Eye Diseases Using Retinal Images from Multiethnic Populations with Diabetes. JAMA 2017, 318, 2211–2223. [Google Scholar] [CrossRef] [PubMed]
- S Stem, M.; W Gardner, T. Neurodegeneration in the Pathogenesis of Diabetic Retinopathy: Molecular Mechanisms and Therapeutic Implications. Curr. Med. Chem. 2013, 20, 3241–3250. [Google Scholar] [CrossRef] [Green Version]
- Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [Green Version]
- Tombran-Tink, J. PEDF in Angiogenic Eye Diseases. Curr. Mol. Med. 2010, 10, 267–278. [Google Scholar] [CrossRef]
- Zafar, S.; Sachdeva, M.; Frankfort, B.J.; Channa, R. Retinal Neurodegeneration as an Early Manifestation of Diabetic Eye Disease and Potential Neuroprotective Therapies. Curr. Diab. Rep. 2019, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T. The age-related macular degeneration as a vascular disease: Contributions to the non-pharmacological interventions and pharmacological therapy arising from its pathogenesis. World J. Pharm. Res. 2016, 5, 91–133. [Google Scholar]
- Liu, Z.; Yu, N.; Holz, F.G.; Yang, F.; Stanzel, B. V Enhancement of Retinal Pigment Epithelial Culture Characteristics and Subretinal Space Tolerance of Scaffolds with 200 Nm Fiber Topography. Biomaterials 2014, 35, 2837–2850. [Google Scholar] [CrossRef]
- Amato, R.; Dal Monte, M.; Lulli, M.; Raffa, V.; Casini, G. Nanoparticle-Mediated Delivery of Neuroprotective Substances for the Treatment of Diabetic Retinopathy. Curr. Neuropharmacol. 2018, 16, 993–1003. [Google Scholar] [CrossRef]
- Sena, D.F.; Lindsley, K. Neuroprotection for Treatment of Glaucoma in Adults. Cochrane Database Syst. Rev. 2017, CD006539. [Google Scholar] [CrossRef]
- Lasseck, J.; Dimitriu, C.; Jehle, T.; Vidal-Sanz, M.; Gozes, I.; Lagreze, W.A. The Neuropeptide NAP Provides Neuroprotection of Retinal Ganglion Cells After Optic Nerve Crush and Retinal Ischemia in Vivo. Investig. Ophthalmol. Vis. Sci. 2007, 48, 565. [Google Scholar]
- Boia, R.; Dias, P.A.N.; Galindo-Romero, C.; Ferreira, H.; Aires, I.D.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Bernardes, R.; Santos, P.F.; de Sousa, H.C. Intraocular Implants Loaded with A3R Agonist Rescue Retinal Ganglion Cells from Ischemic Damage. J. Control. Release 2022, 343, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tatham, A.J.; Weinreb, R.N.; Zangwill, L.M.; Yang, Z.; Zhang, J.Z.; Medeiros, F.A. Relationship between Ganglion Cell Layer Thickness and Estimated Retinal Ganglion Cell Counts in the Glaucomatous Macula. Ophthalmology 2014, 121, 2371–2379. [Google Scholar] [CrossRef] [Green Version]
- Yohannan, J.; Boland, M. V The Evolving Role of the Relationship between Optic Nerve Structure and Function in Glaucoma. Ophthalmology 2017, 124, S66–S70. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Ethier, C.R. Biomechanical Assessment in Models of Glaucomatous Optic Neuropathy. Exp. Eye Res. 2015, 141, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Nickells, R.W.; Howell, G.R.; Soto, I.; John, S.W.M. Under Pressure: Cellular and Molecular Responses during Glaucoma, a Common Neurodegeneration with Axonopathy. Annu. Rev. Neurosci. 2012, 35, 153–179. [Google Scholar] [CrossRef] [Green Version]
- Lusthaus, J.; Goldberg, I. Current Management of Glaucoma. Med. J. Aust. 2019, 210, 180–187. [Google Scholar] [CrossRef]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M.; Group, E.M.G.T. Reduction of Intraocular Pressure and Glaucoma Progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, S.J.; Goldberg, L.D.; Peeples, P.; Walt, J.G.; Bramley, T.J. Current Management of Glaucoma and the Need for Complete Therapy. Am. J. Manag. Care 2008, 14, S20–S27. [Google Scholar] [PubMed]
- Roman, A.J.; Schwartz, S.B.; Aleman, T.S.; Cideciyan, A.V.; Chico, J.D.; Windsor, E.A.M.; Gardner, L.M.; Ying, G.; Smilko, E.E.; Maguire, M.G. Quantifying Rod Photoreceptor-Mediated Vision in Retinal Degenerations: Dark-Adapted Thresholds as Outcome Measures. Exp. Eye Res. 2005, 80, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Acland, G.M.; Aguirre, G.D.; Ray, J.; Zhang, Q.; Aleman, T.S.; Cideciyan, A.V.; Pearce-Kelling, S.E.; Anand, V.; Zeng, Y.; Maguire, A.M. Gene Therapy Restores Vision in a Canine Model of Childhood Blindness. Nat. Genet. 2001, 28, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Langford, M.P.; Redmond, P.; Chanis, R.; Misra, R.P.; Redens, T.B. Glutamate, Excitatory Amino Acid Transporters, Xc− Antiporter, Glutamine Synthetase, and γ-Glutamyltranspeptidase in Human Corneal Epithelium. Curr. Eye Res. 2010, 35, 202–211. [Google Scholar] [CrossRef]
- Boccuni, I.; Fairless, R. Retinal Glutamate Neurotransmission: From Physiology to Pathophysiological Mechanisms of Retinal Ganglion Cell Degeneration. Life 2022, 12, 638. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.M.; Pahlitzsch, M.; Guo, L.; Balendra, S.; Shah, P.; Ravindran, N.; Malaguarnera, G.; Sisa, C.; Shamsher, E.; Hamze, H.; et al. Topical Curcumin Nanocarriers Are Neuroprotective in Eye Disease. Sci. Rep. 2018, 8, 11066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cholkar, K.; Gilger, B.C.; Mitra, A.K. Topical, Aqueous, Clear Cyclosporine Formulation Design for Anterior and Posterior Ocular Delivery. Transl. Vis. Sci. Technol. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- De Guimaraes, T.A.C.; Varela, M.D.; Georgiou, M.; Michaelides, M. Treatments for Dry Age-Related Macular Degeneration: Therapeutic Avenues, Clinical Trials and Future Directions. Br. J. Ophthalmol. 2022, 106, 297–304. [Google Scholar] [CrossRef]
- Heo, H.J.; Lee, C.Y. Protective Effects of Quercetin and Vitamin C against Oxidative Stress-Induced Neurodegeneration. J. Agric. Food Chem. 2004, 52, 7514–7517. [Google Scholar] [CrossRef]
- Borel, P.; Preveraud, D.; Desmarchelier, C. Bioavailability of Vitamin E in Humans: An Update. Nutr. Rev. 2013, 71, 319–331. [Google Scholar] [CrossRef]
- Reboul, E. Absorption of Vitamin A and Carotenoids by the Enterocyte: Focus on Transport Proteins. Nutrients 2013, 5, 3563–3581. [Google Scholar] [CrossRef] [Green Version]
- Teng, H.; Chen, L. Polyphenols and Bioavailability: An Update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Faktorovich, E.G.; Steinberg, R.H.; Yasumura, D.; Matthes, M.T.; LaVail, M.M. Photoreceptor Degeneration in Inherited Retinal Dystrophy Delayed by Basic Fibroblast Growth Factor. Nature 1990, 347, 83–86. [Google Scholar] [CrossRef]
- N Sahni, J.; Angi, M.; Irigoyen, C.; Semeraro, F.; R Romano, M.; Parmeggiani, F. Therapeutic Challenges to Retinitis Pigmentosa: From Neuroprotection to Gene Therapy. Curr. Genom. 2011, 12, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.; Tian, Y.; Zhang, Y.; Lu, Y.-L.; Li, L.-S.; Shi, J.-S. Dendrobium Alkaloids Prevent Aβ25–35-Induced Neuronal and Synaptic Loss via Promoting Neurotrophic Factors Expression in Mice. PeerJ 2016, 4, e2739. [Google Scholar] [CrossRef] [Green Version]
- Leonard, K.C.; Petrin, D.; Coupland, S.G.; Baker, A.N.; Leonard, B.C.; LaCasse, E.C.; Hauswirth, W.W.; Korneluk, R.G.; Tsilfidis, C. XIAP Protection of Photoreceptors in Animal Models of Retinitis Pigmentosa. PLoS ONE 2007, 2, e314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trifunovic, D.; Sahaboglu, A.; Kaur, J.; Mencl, S.; Zrenner, E.; Ueffing, M.; Arango-Gonzalez, B.; Paquet-Durand, F. Neuroprotective Strategies for the Treatment of Inherited Photoreceptor Degeneration. Curr. Mol. Med. 2012, 12, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, A.; Ma, S.; Le, Y.Z.; Hall, M.N.; Rüegg, M.A.; Punzo, C. Activated MTORC1 Promotes Long-Term Cone Survival in Retinitis Pigmentosa Mice. J. Clin. Investig. 2015, 125, 1446–1458. [Google Scholar] [CrossRef] [Green Version]
- Byrne, L.C.; Dalkara, D.; Luna, G.; Fisher, S.K.; Clérin, E.; Sahel, J.-A.; Léveillard, T.; Flannery, J.G. Viral-Mediated RdCVF and RdCVFL Expression Protects Cone and Rod Photoreceptors in Retinal Degeneration. J. Clin. Investig. 2015, 125, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, Y.; Yamagishi, S.; Matsui, T.; Jinnouchi, Y.; Fukami, K.; Imaizumi, T.; Yamakawa, R. Protective Role of Pigment Epithelium-derived Factor (PEDF) in Early Phase of Experimental Diabetic Retinopathy. Diabetes. Metab. Res. Rev. 2009, 25, 678–686. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.; Wu, X.; Liu, Y.; Mu, Z.; Zhang, Y.; Zhang, C.; Fan, Y.; Che, S.; Qian, Z. Gastrodin Regulates GLUR2 Internalization and Reduces BDNF Expression in the Cerebellum of Diabetic Rats. 2022. Available online: https://europepmc.org/article/ppr/ppr506704 (accessed on 25 December 2022.).
- Zhang, X.; Mélik-Parsadaniantz, S.; Baudouin, C.; Réaux-Le Goazigo, A.; Moreau, N. Shh Edding New Light on the Role of Hedgehog Signaling in Corneal Wound Healing. Int. J. Mol. Sci. 2022, 23, 3630. [Google Scholar] [CrossRef]
- Kimura, A.; Namekata, K.; Guo, X.; Harada, C.; Harada, T. Neuroprotection, Growth Factors and BDNF-TrkB Signalling in Retinal Degeneration. Int. J. Mol. Sci. 2016, 17, 1584. [Google Scholar] [CrossRef] [Green Version]
- Mi, S.; Blake Pepinsky, R.; Cadavid, D. Blocking LINGO-1 as a Therapy to Promote CNS Repair: From Concept to the Clinic. CNS Drugs 2013, 27, 493–503. [Google Scholar] [CrossRef] [PubMed]
- So, W.Y.; Leung, P.S. Fibroblast Growth Factor 21 as an Emerging Therapeutic Target for Type 2 Diabetes Mellitus. Med. Res. Rev. 2016, 36, 672–704. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, Z.; Liu, C.-H.; Gong, Y.; Cakir, B.; Liegl, R.; Sun, Y.; Meng, S.S.; Burnim, S.B.; Arellano, I. Fibroblast Growth Factor 21 Protects Photoreceptor Function in Type 1 Diabetic Mice. Diabetes 2018, 67, 974–985. [Google Scholar] [CrossRef] [Green Version]
- Finley, S.D.; Popel, A.S. Predicting the Effects of Anti-Angiogenic Agents Targeting Specific VEGF Isoforms. AAPS J. 2012, 14, 500–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardeljan, C.P.; Ardeljan, D.; Abu-Asab, M.; Chan, C.-C. Inflammation and Cell Death in Age-Related Macular Degeneration: An Immunopathological and Ultrastructural Model. J. Clin. Med. 2014, 3, 1542–1560. [Google Scholar] [CrossRef] [Green Version]
- Perry, T.; Greig, N.H. Enhancing Central Nervous System Endogenous GLP-1 Receptor Pathways for Intervention in Alzheimer’s Disease. Curr. Alzheimer Res. 2005, 2, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Antar, S.A.; Abdo, W.; Taha, R.S.; Farage, A.E.; El-Moselhy, L.E.; Amer, M.E.; Monsef, A.S.A.; Hamid, A.M.A.; Kamel, E.M.; Ahmeda, A.F. Telmisartan Attenuates Diabetic Nephropathy by Mitigating Oxidative Stress and Inflammation, and Upregulating Nrf2/HO-1 Signaling in Diabetic Rats. Life Sci. 2022, 291, 120260. [Google Scholar] [CrossRef]
- Bhatt, U.; Shah, G.; Soni, V. Therapeutic, Protective and Industrial Significances of Anthocyanins: A Review. Avicenna J. Med. Biochem. 2022, 10, 82–93. [Google Scholar]
- Mhatre, S.; Opere, C.A.; Singh, S. Unmet Needs in Glaucoma Therapy: The Potential Role of Hydrogen Sulfide and Its Delivery Strategies. J. Control. Release 2022, 347, 256–269. [Google Scholar] [CrossRef]
- Bhandari, M.; Nguyen, S.; Yazdani, M.; Utheim, T.P.; Hagesaether, E. The Therapeutic Benefits of Nanoencapsulation in Drug Delivery to the Anterior Segment of the Eye: A Systematic Review. Front. Pharmacol. 2022, 13, 903519. [Google Scholar] [CrossRef] [PubMed]
- Akaishi, T.; Kurashima, H.; Odani-Kawabata, N.; Ishida, N.; Nakamura, M. Effects of Repeated Administrations of Tafluprost, Latanoprost, and Travoprost on Optic Nerve Head Blood Flow in Conscious Normal Rabbits. J. Ocul. Pharmacol. Ther. 2010, 26, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Dong, W.-P.; Tang, Y.-B.; Chen, H.-Z.; Cui, Y.-Y.; Bian, X.-L. Huperzine A Lowers Intraocular Pressure via the M3 MAChR and Provides Retinal Neuroprotection via the M1 MAChR: A Promising Agent for the Treatment of Glaucoma. Ann. Transl. Med. 2021, 9, 332. [Google Scholar] [CrossRef]
- Novack, G.D. Ophthalmic Beta-Blockers since Timolol. Surv. Ophthalmol. 1987, 31, 307–327. [Google Scholar] [CrossRef]
- Berson, F.G.; Cohen, H.B.; Foerster, R.J.; Lass, J.H.; Novack, G.D.; Duzman, E. Levobunolol Compared with Timolol for the Long-Term Control of Elevated Intraocular Pressure. Arch. Ophthalmol. 1985, 103, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Shibata-Germanos, S.; Pahlitzsch, M.; Cordeiro, M.F. Current Perspective of Neuroprotection and Glaucoma. Clin. Ophthalmol. 2015, 9, 2109. [Google Scholar]
- Gauthier, A.C.; Liu, J. Focus: The Aging Brain: Neurodegeneration and Neuroprotection in Glaucoma. Yale J. Biol. Med. 2016, 89, 73. [Google Scholar]
- Beal, M.F. Coenzyme Q10 Administration and Its Potential for Treatment of Neurodegenerative Diseases. Biofactors 1999, 9, 261–266. [Google Scholar] [CrossRef]
- Martucci, A.; Nucci, C. Evidence on Neuroprotective Properties of Coenzyme Q10 in the Treatment of Glaucoma. Neural Regen. Res. 2019, 14, 197. [Google Scholar]
- Davis, B.M.; Tian, K.; Pahlitzsch, M.; Brenton, J.; Ravindran, N.; Butt, G.; Malaguarnera, G.; Normando, E.M.; Guo, L.; Cordeiro, M.F. Topical Coenzyme Q10 Demonstrates Mitochondrial-Mediated Neuroprotection in a Rodent Model of Ocular Hypertension. Mitochondrion 2017, 36, 114–123. [Google Scholar] [CrossRef]
- Nakajima, Y.; Inokuchi, Y.; Nishi, M.; Shimazawa, M.; Otsubo, K.; Hara, H. Coenzyme Q10 Protects Retinal Cells against Oxidative Stress in Vitro and in Vivo. Brain Res. 2008, 1226, 226–233. [Google Scholar] [CrossRef]
- Lipton, S.A. Possible Role for Memantine in Protecting Retinal Ganglion Cells from Glaucomatous Damage. Surv. Ophthalmol. 2003, 48, S38–S46. [Google Scholar] [CrossRef] [PubMed]
- Roberti, G.; Tanga, L.; Michelessi, M.; Quaranta, L.; Parisi, V.; Manni, G.; Oddone, F. Cytidine 5′-Diphosphocholine (Citicoline) in Glaucoma: Rationale of Its Use, Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2015, 16, 28401–28417. [Google Scholar] [CrossRef] [Green Version]
- Parisi, V.; Oddone, F.; Ziccardi, L.; Roberti, G.; Coppola, G.; Manni, G. Citicoline and Retinal Ganglion Cells: Effects on Morphology and Function. Curr. Neuropharmacol. 2018, 16, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Chiosi, F.; Di Marco, R.; Costagliola, C.; Scapagnini, G. Cytoprotective Effects of Citicoline and Homotaurine against Glutamate and High Glucose Neurotoxicity in Primary Cultured Retinal Cells. Oxid. Med. Cell. Longev. 2017, 2017, 2825703. [Google Scholar] [CrossRef] [PubMed]
- Cvenkel, B.; Kolko, M. Current Medical Therapy and Future Trends in the Management of Glaucoma Treatment. J. Ophthalmol. 2020, 2020, 6138132. [Google Scholar] [CrossRef]
- Araie, M.; Mayama, C. Use of Calcium Channel Blockers for Glaucoma. Prog. Retin. Eye Res. 2011, 30, 54–71. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Payne, S.C.; Bartlett, C.A.; Evill, L.; Harvey, A.R.; Dunlop, S.A. Secondary Retinal Ganglion Cell Death and the Neuroprotective Effects of the Calcium Channel Blocker Lomerizine. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5456–5462. [Google Scholar] [CrossRef] [Green Version]
- Mackay, D.D.; Atkins, E.J.; Newman, N.J.; Biousse, V. Nonarteritic Anterior Ischemic Optic Neuropathy: Untreatable at Present? Expert Rev. Ophthalmol. 2013, 8, 363–374. [Google Scholar] [CrossRef]
- Shew, W.; Wang, M.T.M.; Danesh-Meyer, H.V. Nonarteritic Anterior Ischemic Optic Neuropathy After Cataract Surgery: A Systematic Review and Meta-Analysis. J. Neuro-Ophthalmol. 2022, 43, 17–28. [Google Scholar] [CrossRef]
- Fischbarg, J. The Biology of the Eye; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 0080476090. [Google Scholar]
- Sasaki, H.; Yamamura, K.; Nishida, K.; Nakamura, J.; Ichikawa, M. Delivery of Drugs to the Eye by Topical Application. Prog. Retin. Eye Res. 1996, 15, 583–620. [Google Scholar] [CrossRef]
- Chien, D.-S.; Sasaki, H.; Bundgaard, H.; Buur, A.; Lee, V.H.L. Role of Enzymatic Lability in the Corneal and Conjunctival Penetration of Timolol Ester Prodrugs in the Pigmented Rabbit. Pharm. Res. 1991, 8, 728–733. [Google Scholar] [CrossRef]
- DiSandro, G.; Samudre, S.S.; Williams, P.B.; Billy, M.; Frazier, M.; Crouch, E.R., Jr.; Lattanzio, F.A., Jr. Ocular Pharmacokinetics of Lipophilic and Hydrophilic Synthetic Cannabinoids in an Artificially Perfused Rat Eye Model. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1209. [Google Scholar]
- Peng, C.-C.; Kim, J.; Chauhan, A. Extended Delivery of Hydrophilic Drugs from Silicone-Hydrogel Contact Lenses Containing Vitamin E Diffusion Barriers. Biomaterials 2010, 31, 4032–4047. [Google Scholar] [CrossRef]
- Roberti, G.; Tanga, L.; Parisi, V.; Sampalmieri, M.; Centofanti, M.; Manni, G. A Preliminary Study of the Neuroprotective Role of Citicoline Eye Drops in Glaucomatous Optic Neuropathy. Indian J. Ophthalmol. 2014, 62, 549–553. [Google Scholar] [CrossRef]
- Lee, S.J.; He, W.; Robinson, S.B.; Robinson, M.R.; Csaky, K.G.; Kim, H. Evaluation of Clearance Mechanisms with Transscleral Drug Delivery. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5205–5212. [Google Scholar] [CrossRef]
- Dey, S.; Patel, J.; Anand, B.S.; Jain-Vakkalagadda, B.; Kaliki, P.; Pal, D.; Ganapathy, V.; Mitra, A.K. Molecular Evidence and Functional Expression of P-Glycoprotein (MDR1) in Human and Rabbit Cornea and Corneal Epithelial Cell Lines. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2909–2918. [Google Scholar] [CrossRef] [Green Version]
- Vellonen, K.-S.; Mannermaa, E.; Turner, H.; Häkli, M.; Wolosin, J.M.; Tervo, T.; Honkakoski, P.; Urtti, A. Effluxing ABC Transporters in Human Corneal Epithelium. J. Pharm. Sci. 2010, 99, 1087–1098. [Google Scholar] [CrossRef] [Green Version]
- Duncan, K.G.; Hosseini, K.; Bailey, K.R.; Yang, H.; Lowe, R.J.; Matthes, M.T.; Kane, J.P.; LaVail, M.M.; Schwartz, D.M.; Duncan, J.L. Expression of Reverse Cholesterol Transport Proteins ATP-Binding Cassette A1 (ABCA1) and Scavenger Receptor BI (SR-BI) in the Retina and Retinal Pigment Epithelium. Br. J. Ophthalmol. 2009, 93, 1116–1120. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.-Y.; Hao, J.-L.; Wang, S.; Zheng, Y.; Zhang, W.-S. Nanoparticles in the Ocular Drug Delivery. Int. J. Ophthalmol. 2013, 6, 390. [Google Scholar]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Ruponen, M.; Urtti, A. Undefined Role of Mucus as a Barrier in Ocular Drug Delivery. Eur. J. Pharm. Biopharm. 2015, 96, 442–446. [Google Scholar] [CrossRef]
- Ahmad, H.; Singh, S.V.; Awasthi, Y.C. Inhibition of Bovine Lens Glutathione S-Transferases by Hematin, Bilirubin, and Bromosulfophthalein. Lens Eye Toxic. Res. 1991, 8, 431–440. [Google Scholar] [PubMed]
- Duvvuri, S.; Majumdar, S.; Mitra, A.K. Role of Metabolism in Ocular Drug Delivery. Curr. Drug Metab. 2004, 5, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Rimpelä, A.-K.; Reinisalo, M.; Hellinen, L.; Grazhdankin, E.; Kidron, H.; Urtti, A.; Del Amo, E.M. Implications of Melanin Binding in Ocular Drug Delivery. Adv. Drug Deliv. Rev. 2018, 126, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Salminen, L.; Urtti, A. Disposition of Ophthalmic Timolol in Treated and Untreated Rabbit Eyes. A Multiple and Single Dose Study. Exp. Eye Res. 1984, 38, 203–206. [Google Scholar] [CrossRef]
- Salminen, L.; Imre, G.; Huupponen, R. The Effect of Ocular Pigmentation on Intraocular Pressure Response to Timolol. Acta Ophthalmol. Suppl. 1985, 173, 15–18. [Google Scholar] [CrossRef]
- Maekawa, Y.; Hasegawa, S.; Ishizuka, T.; Shiosakai, K.; Ishizuka, H. Pharmacokinetics and Bioequivalence of Memantine Tablet and a New Dry Syrup Formulation in Healthy Japanese Males: Two Single-Dose Crossover Studies. Adv. Ther. 2019, 36, 2930–2940. [Google Scholar] [CrossRef] [Green Version]
- Morris, P.J.; Burke, R.D.; Sharma, A.K.; Lynch, D.C.; Lemke-Boutcher, L.E.; Mathew, S.; Elayan, I.; Rao, D.B.; Gould, T.D.; Zarate Jr, C.A. A Comparison of the Pharmacokinetics and NMDAR Antagonism-Associated Neurotoxicity of Ketamine, (2R, 6R)-Hydroxynorketamine and MK-801. Neurotoxicol. Teratol. 2021, 87, 106993. [Google Scholar] [CrossRef]
- Perdigão, A.P.L.; de Jesus Antunes, N.; Juni, L.T.; de Freitas, N.L.; Rojas-Moscoso, J.; Corrêa, S.V.M.; da Costa, R.C.; Moreno, R.A.; Mendes, G.D.; De Nucci, G. Pharmacokinetics of Riluzole in Beagle Dogs. Drug Res. 2019, 69, 40–45. [Google Scholar] [CrossRef]
- Tamhane, M.; Luu, K.T.; Attar, M. Ocular Pharmacokinetics of Brimonidine Drug Delivery System in Monkeys and Translational Modeling for Selection of Dose and Frequency in Clinical Trials. J. Pharmacol. Exp. Ther. 2021, 378, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, I.; Heaton, R.A.; Mantle, D. Disorders of Human Coenzyme Q10 Metabolism: An Overview. Int. J. Mol. Sci. 2020, 21, 6695. [Google Scholar] [CrossRef] [PubMed]
- Secades, J.J. Citicolina: Revisión Farmacológica y Clínica, Actualización 2010. Rev. Neurol. 2011, 52, 1–62. [Google Scholar] [CrossRef]
- Xu, Y.; Yi, Z.; Li, X.; Li, D.; Pan, L.; Dai, Y.; Zhong, X.; Yan, J.; Xu, P.; Xu, S. Pharmacokinetics of Flunarizine Hydrochloride After Single Oral Doses in Healthy Subjects: Bioequivalence Study and Food Effects. Clin. Pharmacol. Drug Dev. 2022, 11, 341–347. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, T.; Song, G.; Hu, Y.; Liang, J. Determination of Lomerizine in Human Plasma by Liquid Chromatography/Tandem Mass Spectrometry and Its Application to a Pharmacokinetic Study. J. Chromatogr. B 2014, 947, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Tynan, B.E.; Papich, M.G.; Kerl, M.E.; Cohn, L.A. Pharmacokinetics of Minocycline in Domestic Cats. J. Feline Med. Surg. 2016, 18, 257–263. [Google Scholar] [CrossRef]
- Eisenhauer, B.; Natoli, S.; Liew, G.; Flood, V.M. Lutein and Zeaxanthin—Food Sources, Bioavailability and Dietary Variety in Age-Related Macular Degeneration Protection. Nutrients 2017, 9, 120. [Google Scholar] [CrossRef]
- Eisvand, F.; Razavi, B.M.; Hosseinzadeh, H. The Effects of Ginkgo Biloba on Metabolic Syndrome: A Review. Phyther. Res. 2020, 34, 1798–1811. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [Green Version]
- Gabrielsson, L.; Mattsson, S.; Fowler, C.J. Palmitoylethanolamide for the Treatment of Pain: Pharmacokinetics, Safety and Efficacy. Br. J. Clin. Pharmacol. 2016, 82, 932–942. [Google Scholar] [CrossRef] [Green Version]
- Harpsøe, N.G.; Andersen, L.P.H.; Gögenur, I.; Rosenberg, J. Clinical Pharmacokinetics of Melatonin: A Systematic Review. Eur. J. Clin. Pharmacol. 2015, 71, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.U.; Bjerg, M.; Ulaganathan, N.; Holm, R. Oral and Intravenous Pharmacokinetics of Taurine in Sprague-dawley Rats: The Influence of Dose and the Possible Involvement of the Proton-coupled Amino Acid Transporter, PAT1, in Oral Taurine Absorption. Physiol. Rep. 2017, 5, e13467. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sang, S. Metabolism and Pharmacokinetics of Resveratrol and Pterostilbene. Biofactors 2018, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-J.; Jiang, D.; Tian, L.-L.; Yin, J.-J.; Huang, J.-M.; Weng, W.-Y. Intestinal Permeability of Forskolin by in Situ Single Pass Perfusion in Rats. Planta Med. 2012, 78, 698–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adiwidjaja, J.; McLachlan, A.J.; Boddy, A. V Curcumin as a Clinically-Promising Anti-Cancer Agent: Pharmacokinetics and Drug Interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 953–972. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Pharmacokinetic Properties of Saffron and Its Active Components. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 383–390. [Google Scholar] [CrossRef]
- Fayyaz, A.; Vellonen, K.-S.; Ranta, V.-P.; Toropainen, E.; Reinisalo, M.; Valtari, A.; Puranen, J.; Ricci, G.D.; Heikkinen, E.M.; Gardner, I.; et al. Ocular Pharmacokinetics of Atenolol, Timolol and Betaxolol Cocktail: Tissue Exposures in the Rabbit Eye. Eur. J. Pharm. Biopharm. 2021, 166, 155–162. [Google Scholar] [CrossRef]
- Lalak, N.J.; Morris, D.L. Azithromycin Clinical Pharmacokinetics. Clin. Pharmacokinet. 1993, 25, 370–374. [Google Scholar] [CrossRef]
- Foulds, G. Pharmacokinetics of Sulbactam/Ampicillin in Humans: A Review. Rev. Infect. Dis. 1986, 8 (Suppl. 5), S503–S511. [Google Scholar] [CrossRef]
- Wolfson, J.S.; Hooper, D.C. Comparative Pharmacokinetics of Ofloxacin and Ciprofloxacin. Am. J. Med. 1989, 87, 31S–36S. [Google Scholar]
- Gonzalez-Pizarro, R.; Carvajal-Vidal, P.; Halbault Bellowa, L.; Calpena, A.C.; Espina, M.; García, M.L. In-Situ Forming Gels Containing Fluorometholone-Loaded Polymeric Nanoparticles for Ocular Inflammatory Conditions. Colloids Surf. B Biointerfaces 2019, 175, 365–374. [Google Scholar] [CrossRef]
- Schulz, J.; Kluwe, F.; Mikus, G.; Michelet, R.; Kloft, C. Novel Insights into the Complex Pharmacokinetics of Voriconazole: A Review of Its Metabolism. Drug Metab. Rev. 2019, 51, 247–265. [Google Scholar] [CrossRef]
- Yilmaz, T.; Cordero-Coma, M.; Federici, T.J. Pharmacokinetics of Triamcinolone Acetonide for the Treatment of Macular Edema. Expert Opin. Drug Metab. Toxicol. 2011, 7, 1327–1335. [Google Scholar] [CrossRef]
- Zhao, X.; Tong, Y.; Wang, X.; Zhang, X.; Chen, S.; Lu, H. Comparison of the Ocular Penetration and Pharmacokinetics Between Natamycin and Voriconazole After Topical Instillation in Rabbits. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2018, 34, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Abshagen, U.; Rennekamp, H.; Luszpinski, G. Pharmacokinetics of Spironolactone in Man. Naunyn. Schmiedebergs Arch. Pharmacol. 1976, 296, 37–45. [Google Scholar] [CrossRef]
- Debruyne, D.; Ryckelynck, J.P. Clinical Pharmacokinetics of Fluconazole. Clin. Pharmacokinet. 1993, 24, 10–27. [Google Scholar] [CrossRef]
- Greenberg, R.G.; Landersdorfer, C.B.; Rivera-Chaparro, N.; Harward, M.; Conrad, T.; Nakamura, A.; Kirkpatrick, C.M.; Gu, K.; Ghazaryhan, V.; Osborn, B.; et al. Population Pharmacokinetics of Moxifloxacin in Children. Paediatr. Drugs 2022, 24, 163–173. [Google Scholar] [CrossRef]
- Kalam, M.N.; Rasool, M.F.; Rehman, A.U.; Ahmed, N. Clinical Pharmacokinetics of Propranolol Hydrochloride: A Review. Curr. Drug Metab. 2020, 21, 89–105. [Google Scholar] [CrossRef]
- Dohil, R.; Cabrera, B.L.; Gangoiti, J.A.; Barshop, B.A.; Rioux, P. Pharmacokinetics of Cysteamine Bitartrate Following Intraduodenal Delivery. Fundam. Clin. Pharmacol. 2014, 28, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Fahr, A. Cyclosporin Clinical Pharmacokinetics. Clin. Pharmacokinet. 1993, 24, 472–495. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Sun, L.; DuBois, D.C.; Almon, R.R.; Meng, S.; Jusko, W.J. Physiologically Based Pharmacokinetics of Dexamethasone in Rats. Drug Metab. Dispos. 2020, 48, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Mallandrich, M.; Calpena, A.C.; Clares, B.; Parra, A.; García, M.L.; Soriano, J.L.; Fernández-Campos, F. Nano-Engineering of Ketorolac Tromethamine Platforms for Ocular Treatment of Inflammatory Disorders. Nanomedicine 2021, 16, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Serpe, L.; Foglietta, F.; Muntoni, E.; Gallarate, M.; Del Pozo Rodriguez, A.; Solinis, M.A. Application of Lipid Nanoparticles to Ocular Drug Delivery. Expert Opin. Drug Deliv. 2016, 13, 1743–1757. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Ahmadi, F.; Hashemi, S.M.H.; Babaei, A.; Yaddollahi, S.; Rostamkalaei, S.S.; Asare-Addo, K.; Nokhodchi, A. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: A Review of the Methods of Manufacture and Routes of Administration. Pharm. Dev. Technol. 2022, 27, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, D.; Sarpietro, M.G.; Carbone, C.; Panico, A.; Campisi, A.; Siciliano, E.A.; Sposito, G.; Castelli, F.; Puglia, C. Curcumin Containing PEGylated Solid Lipid Nanoparticles for Systemic Administration: A Preliminary Study. Molecules 2020, 25, 2991. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Bhanderi, B.; Soniwala, M.; Chavda, J. Lutein-Loaded Solid Lipid Nanoparticles for Ocular Delivery: Statistical Optimization and Ex Vivo Evaluation. J. Pharm. Innov. 2022, 17, 584–598. [Google Scholar] [CrossRef]
- El-Salamouni, N.S.; Farid, R.M.; El-Kamel, A.H.; El-Gamal, S.S. Nanostructured Lipid Carriers for Intraocular Brimonidine Localisation: Development, in-Vitro and in-Vivo Evaluation. J. Microencapsul. 2018, 35, 102–113. [Google Scholar] [CrossRef]
- Liu, C.-H.; Huang, Y.-C.; Jhang, J.-W.; Liu, Y.-H.; Wu, W.-C. Quercetin Delivery to Porcine Cornea and Sclera by Solid Lipid Nanoparticles and Nanoemulsion. RSC Adv. 2015, 5, 100923–100933. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Ye, T.; Chen, F.; Yu, S.; Chen, J.; Yang, X.; Yang, N.; Zhang, J.; Liu, J.; et al. Nanostructured Lipid Carrier Surface Modified with Eudragit RS 100 and Its Potential Ophthalmic Functions. Int. J. Nanomed. 2014, 9, 4305–4315. [Google Scholar] [CrossRef]
- Ana, R.D.; Fonseca, J.; Karczewski, J.; Silva, A.M.; Zielińska, A.; Souto, E.B. Lipid-Based Nanoparticulate Systems for the Ocular Delivery of Bioactives with Anti-Inflammatory Properties. Int. J. Mol. Sci. 2022, 23, 12102. [Google Scholar] [CrossRef]
- Attama, A.A.; Reichl, S.; Müller-Goymann, C.C. Sustained Release and Permeation of Timolol from Surface-Modified Solid Lipid Nanoparticles through Bioengineered Human Cornea. Curr. Eye Res. 2009, 34, 698–705. [Google Scholar] [CrossRef]
- Dang, H.; Dong, C.; Zhang, L. Sustained Latanoprost Release from PEGylated Solid Lipid Nanoparticle-Laden Soft Contact Lens to Treat Glaucoma. Pharm. Dev. Technol. 2022, 27, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Wadetwar, R.N.; Agrawal, A.R.; Kanojiya, P.S. In Situ Gel Containing Bimatoprost Solid Lipid Nanoparticles for Ocular Delivery: In-Vitro and Ex-Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2020, 56, 101575. [Google Scholar] [CrossRef]
- Tatke, A.; Dudhipala, N.; Janga, K.Y.; Balguri, S.P.; Avula, B.; Jablonski, M.M.; Majumdar, S. In Situ Gel of Triamcinolone Acetonide-Loaded Solid Lipid Nanoparticles for Improved Topical Ocular Delivery: Tear Kinetics and Ocular Disposition Studies. Nanomaterials 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Xu, S.; Yu, S.; Li, J.; Tan, G.; Li, S.; Pan, W. A Hybrid Genipin-Cross-Linked Hydrogel/Nanostructured Lipid Carrier for Ocular Drug Delivery: Cellular, Ex Vivo, and in Vivo Evaluation. ACS Biomater. Sci. Eng. 2020, 6, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Nakazawa, Y.; Ito, Y.; Kanai, K.; Okamoto, N.; Shimomura, Y. A Nanoparticle-Based Ophthalmic Formulation of Dexamethasone Enhances Corneal Permeability of the Drug and Prolongs Its Corneal Residence Time. Biol. Pharm. Bull. 2017, 40, 1055–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Lian, Y.; Fang, Q.; Liu, L.; Zhang, J.; Li, J. Hyaluronic-Acid-Modified Lipid-Polymer Hybrid Nanoparticles as an Efficient Ocular Delivery Platform for Moxifloxacin Hydrochloride. Int. J. Biol. Macromol. 2018, 116, 1026–1036. [Google Scholar] [CrossRef]
- Waite, D.; Adrianto, F.M.; Annuyanti, F.; So, Y.; Zhang, W.; Wang, S.; Wu, Y.; Wang, Y.; Singh, T.R.R. Long-Acting Drug Delivery Systems for Ocular Therapies. In Long-Acting Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 61–81. [Google Scholar]
- Shen, Y.; Tu, J. Preparation and Ocular Pharmacokinetics of Ganciclovir Liposomes. AAPS J. 2007, 9, E371–E377. [Google Scholar] [CrossRef] [Green Version]
- Chetoni, P.; Monti, D.; Tampucci, S.; Matteoli, B.; Ceccherini-Nelli, L.; Subissi, A.; Burgalassi, S. Liposomes as a Potential Ocular Delivery System of Distamycin A. Int. J. Pharm. 2015, 492, 120–126. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth Liposomes: Review of the Basic Science, Rationale, and Clinical Applications, Existing and Potential. Int. J. Nanomed. 2006, 1, 297. [Google Scholar]
- Ibrahim, A.E.; Shafaa, M.W.; Khedr, M.H.; Rashed, R.F. Comparative Study between Lutein and Its Liposomal Form on Cisplatin-Induced Retinal Injury in Rabbits. Cutan. Ocul. Toxicol. 2019, 38, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.S.; Moon, M.J.; Thomas, R.G.; Kim, S.Y.; Lee, J.S.; Jeong, Y.Y.; Park, I.-K.; Park, S.W. Intravitreal Injection of Liposomes Loaded with a Histone Deacetylase Inhibitor Promotes Retinal Ganglion Cell Survival in a Mouse Model of Optic Nerve Crush. Int. J. Mol. Sci. 2020, 21, 9297. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Wei, Y.; Wu, Q.; Zhou, K.; Liu, T.; Zhang, Y.; Jiang, N.; Xiao, W.; Chen, J.; Liu, Q.; et al. Liposomes for Effective Drug Delivery to the Ocular Posterior Chamber. J. Nanobiotechnol. 2019, 17, 64. [Google Scholar] [CrossRef] [Green Version]
- Nagarwal, R.C.; Kant, S.; Singh, P.N.; Maiti, P.; Pandit, J.K. Polymeric Nanoparticulate System: A Potential Approach for Ocular Drug Delivery. J. Control. Release 2009, 136, 2–13. [Google Scholar] [CrossRef] [PubMed]
- De Campos, A.M.; Diebold, Y.; Carvalho, E.L.S.; Sánchez, A.; José Alonso, M. Chitosan Nanoparticles as New Ocular Drug Delivery Systems: In Vitro Stability, in Vivo Fate, and Cellular Toxicity. Pharm. Res. 2004, 21, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, D.; Xu, Y.; Zhu, Q. Hyaluronic Acid in Ocular Drug Delivery. Carbohydr. Polym. 2021, 264, 118006. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.A.; El-Feky, G.S.; Kamel, R.; Awad, G.E.A. Chitosan/Sulfobutylether-β-Cyclodextrin Nanoparticles as a Potential Approach for Ocular Drug Delivery. Int. J. Pharm. 2011, 413, 229–236. [Google Scholar] [CrossRef]
- Bessone, C.D.V.; Martinez, S.M.; Luna, J.D.; Marquez, M.A.; Ramírez, M.L.; Allemandi, D.A.; Carpentieri, Á.R.; Quinteros, D.A. Neuroprotective Effect of Melatonin Loaded in Ethylcellulose Nanoparticles Applied Topically in a Retinal Degeneration Model in Rabbits. Exp. Eye Res. 2020, 200, 108222. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Egea, M.A.; Davis, B.M.; Guo, L.; Espina, M.; Silva, A.M.; Calpena, A.C.; Souto, E.M.B.; Ravindran, N.; Ettcheto, M.; et al. Memantine-Loaded PEGylated Biodegradable Nanoparticles for the Treatment of Glaucoma. Small 2018, 14, 1701808. [Google Scholar] [CrossRef]
- Yang, H.; Tyagi, P.; Kadam, R.S.; Holden, C.A.; Kompella, U.B. Hybrid Dendrimer Hydrogel/PLGA Nanoparticle Platform Sustains Drug Delivery for One Week and Antiglaucoma Effects for Four Days Following One-Time Topical Administration. ACS Nano 2012, 6, 7595–7606. [Google Scholar] [CrossRef]
- Sharma, P.K.; Chauhan, M.K. Optimization and Characterization of Brimonidine Tartrate Nanoparticles-Loaded In Situ Gel for the Treatment of Glaucoma. Curr. Eye Res. 2021, 46, 1703–1716. [Google Scholar] [CrossRef]
- Cardoso, C.O.; Ferreira-Nunes, R.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. In Situ Gelling Microemulsion for Topical Ocular Delivery of Moxifloxacin and Betamethasone. J. Mol. Liq. 2022, 360, 119559. [Google Scholar] [CrossRef]
- Dandamudi, M.; McLoughlin, P.; Behl, G.; Rani, S.; Coffey, L.; Chauhan, A.; Kent, D.; Fitzhenry, L. Chitosan-Coated PLGA Nanoparticles Encapsulating Triamcinolone Acetonide as a Potential Candidate for Sustained Ocular Drug Delivery. Pharmaceutics 2021, 13, 1590. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.; Paliwal, R.; Paliwal, S.R.; Vyas, S.P. Hyaluronic Acid Modified Chitosan Nanoparticles for Effective Management of Glaucoma: Development, Characterization, and Evaluation. J. Drug Target. 2010, 18, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Bharadwaj, S.; Lee, K.E.; Kang, S.G. Therapeutic Nanoemulsions in Ophthalmic Drug Administration: Concept in Formulations and Characterization Techniques for Ocular Drug Delivery. J. Control. Release 2020, 328, 895–916. [Google Scholar] [CrossRef]
- Ammar, H.O.; Salama, H.A.; Ghorab, M.; Mahmoud, A.A. Development of Dorzolamide Hydrochloride in Situ Gel Nanoemulsion for Ocular Delivery. Drug Dev. Ind. Pharm. 2010, 36, 1330–1339. [Google Scholar] [CrossRef]
- Daull, P.; Lallemand, F.; Garrigue, J.-S. Benefits of Cetalkonium Chloride Cationic Oil-in-Water Nanoemulsions for Topical Ophthalmic Drug Delivery. J. Pharm. Pharmacol. 2014, 66, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Garg, V.; Jain, G.K.; Nirmal, J.; Kohli, K. Topical Tacrolimus Nanoemulsion, a Promising Therapeutic Approach for Uveitis. Med. Hypotheses 2013, 81, 901–904. [Google Scholar] [CrossRef]
- Choradiya, B.R.; Patil, S.B. A Comprehensive Review on Nanoemulsion as an Ophthalmic Drug Delivery System. J. Mol. Liq. 2021, 339, 116751. [Google Scholar] [CrossRef]
- Silva, R.; Vilas-Boas, V.; Carmo, H.; Dinis-Oliveira, R.J.; Carvalho, F.; de Lourdes Bastos, M.; Remiao, F. Modulation of P-Glycoprotein Efflux Pump: Induction and Activation as a Therapeutic Strategy. Pharmacol. Ther. 2015, 149, 1–123. [Google Scholar] [CrossRef]
- Lim, C.; Kim, D.; Sim, T.; Hoang, N.H.; Lee, J.W.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Preparation and Characterization of a Lutein Loading Nanoemulsion System for Ophthalmic Eye Drops. J. Drug Deliv. Sci. Technol. 2016, 36, 168–174. [Google Scholar] [CrossRef]
- Fialho, S.L.; Da Silva-Cunha, A. New Vehicle Based on a Microemulsion for Topical Ocular Administration of Dexamethasone. Clin. Exp. Ophthalmol. 2004, 32, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Dang, H.; Huang, C.; Sheng, Y. Timolol Loaded Microemulsion Laden Silicone Contact Lens to Manage Glaucoma: In Vitro and in Vivo Studies. J. Dispers. Sci. Technol. 2021, 42, 742–750. [Google Scholar] [CrossRef]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric Micelles for Ocular Drug Delivery: From Structural Frameworks to Recent Preclinical Studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cholkar, K.; Patel, A.; Dutt Vadlapudi, A.; K Mitra, A. Novel Nanomicellar Formulation Approaches for Anterior and Posterior Segment Ocular Drug Delivery. Recent Patents Nanomed. 2012, 2, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Özsoy, Y.; Güngör, S.; Kahraman, E.; Durgun, M.E. Polymeric Micelles as a Novel Carrier for Ocular Drug Delivery. In Nanoarchitectonics in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–117. [Google Scholar]
- Xu, L.; Xu, X.; Chen, H.; Li, X. Ocular Biocompatibility and Tolerance Study of Biodegradable Polymeric Micelles in the Rabbit Eye. Colloids Surf. B Biointerfaces 2013, 112, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Durgun, M.E.; Güngör, S.; Özsoy, Y. Micelles: Promising Ocular Drug Carriers for Anterior and Posterior Segment Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zong, R.; Bao, X.; Zheng, X.; Cui, H.; Liu, Z.; Zhou, Y. Oxidative Stress Suppresses Cellular Autophagy in Corneal Epithelium. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3286–3293. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Lin, G.; Xie, Y.; Ma, P.; Li, G.; Meng, Q.; Wu, T. Preformulation Studies of Myricetin: A Natural Antioxidant Flavonoid. Pharmazie 2014, 69, 19–26. [Google Scholar]
- Sun, F.; Zheng, Z.; Lan, J.; Li, X.; Li, M.; Song, K.; Wu, X. New Micelle Myricetin Formulation for Ocular Delivery: Improved Stability, Solubility, and Ocular Anti-Inflammatory Treatment. Drug Deliv. 2019, 26, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhang, L.; Li, R.; Yan, M. New Resveratrol Micelle Formulation for Ocular Delivery: Characterization and In Vitro/In Vivo Evaluation. Drug Dev. Ind. Pharm. 2020, 46, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Lancina III, M.G.; Yang, H. Dendrimers for Ocular Drug Delivery. Can. J. Chem. 2017, 95, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Sun, K.; Mu, H.; Liang, N.; Liu, Y.; Yao, C.; Liang, R.; Wang, A. Preparation and Characterization of Puerarin–Dendrimer Complexes as an Ocular Drug Delivery System. Drug Dev. Ind. Pharm. 2010, 36, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Holden, C.A.; Tyagi, P.; Thakur, A.; Kadam, R.; Jadhav, G.; Kompella, U.B.; Yang, H. Polyamidoamine Dendrimer Hydrogel for Enhanced Delivery of Antiglaucoma Drugs. Nanomedicine 2012, 8, 776–783. [Google Scholar] [CrossRef]
- Durairaj, C.; Kadam, R.S.; Chandler, J.W.; Hutcherson, S.L.; Kompella, U.B. Nanosized Dendritic Polyguanidilyated Translocators for Enhanced Solubility, Permeability, and Delivery of Gatifloxacin. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5804–5816. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Chau, Y. Enhanced Delivery of SiRNA to Retinal Ganglion Cells by Intravitreal Lipid Nanoparticles of Positive Charge. Mol. Pharm. 2020, 18, 377–385. [Google Scholar] [CrossRef]
- McNeil, S.E. Characterization of Nanoparticles Intended for Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2011; Volume 697. [Google Scholar]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zein, R.; Alghoraibi, I.; Soukkarieh, C.; Salman, A.; Alahmad, A. In-Vitro Anticancer Activity against Caco-2 Cell Line of Colloidal Nano Silver Synthesized Using Aqueous Extract of Eucalyptus Camaldulensis Leaves. Heliyon 2020, 6, e04594. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Holback, H.; Liu, K.C.; Abouelmagd, S.A.; Park, J.; Yeo, Y. Nanoparticle Characterization: State of the Art, Challenges, and Emerging Technologies. Mol. Pharm. 2013, 10, 2093–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chariou, P.L.; Ortega-Rivera, O.A.; Steinmetz, N.F. Nanocarriers for the Delivery of Medical, Veterinary, and Agricultural Active Ingredients. ACS Nano 2020, 14, 2678–2701. [Google Scholar] [CrossRef]
- Wang, W.; Ye, Z.; Gao, H.; Ouyang, D. Computational Pharmaceutics-A New Paradigm of Drug Delivery. J. Control. Release 2021, 338, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, C.; Pande, S.; Sagathia, V.; Ranch, K.; Beladiya, J.; Boddu, S.H.S.; Jacob, S.; Al-Tabakha, M.M.; Hassan, N.; Shahwan, M. Nanocarriers for the Delivery of Neuroprotective Agents in the Treatment of Ocular Neurodegenerative Diseases. Pharmaceutics 2023, 15, 837. https://doi.org/10.3390/pharmaceutics15030837
Patel C, Pande S, Sagathia V, Ranch K, Beladiya J, Boddu SHS, Jacob S, Al-Tabakha MM, Hassan N, Shahwan M. Nanocarriers for the Delivery of Neuroprotective Agents in the Treatment of Ocular Neurodegenerative Diseases. Pharmaceutics. 2023; 15(3):837. https://doi.org/10.3390/pharmaceutics15030837
Chicago/Turabian StylePatel, Chirag, Sonal Pande, Vrunda Sagathia, Ketan Ranch, Jayesh Beladiya, Sai H. S. Boddu, Shery Jacob, Moawia M. Al-Tabakha, Nageeb Hassan, and Moyad Shahwan. 2023. "Nanocarriers for the Delivery of Neuroprotective Agents in the Treatment of Ocular Neurodegenerative Diseases" Pharmaceutics 15, no. 3: 837. https://doi.org/10.3390/pharmaceutics15030837
APA StylePatel, C., Pande, S., Sagathia, V., Ranch, K., Beladiya, J., Boddu, S. H. S., Jacob, S., Al-Tabakha, M. M., Hassan, N., & Shahwan, M. (2023). Nanocarriers for the Delivery of Neuroprotective Agents in the Treatment of Ocular Neurodegenerative Diseases. Pharmaceutics, 15(3), 837. https://doi.org/10.3390/pharmaceutics15030837