Functionalization of 3D-Printed Titanium Scaffolds with Elastin-like Recombinamers to Improve Cell Colonization and Osteoinduction
Abstract
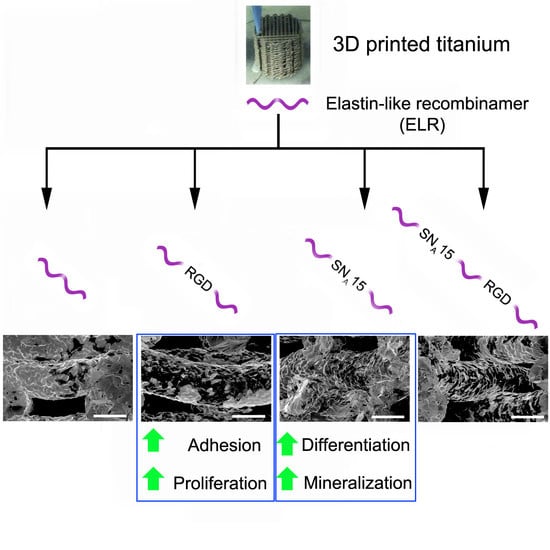
:1. Introduction
2. Materials and Methods
2.1. Printing of Scaffolds
2.2. Synthesis and Characterization of Elastin-like Recombinamers
2.3. Scaffold Functionalization
2.4. Characterization of ELR Functionalization
2.5. Cell Culture and Maintenance
2.6. Cell Adhesion and Proliferation
2.7. Cell Colonization and Morphology
2.8. Cell Differentiation and Mineralization
2.9. Statistical Analysis
3. Results and Discussion
3.1. ELR Synthesis and Characterization
3.2. Scaffolds Functionalization
3.3. Cell Adhesion and Proliferation
3.4. Cell Colonization
3.5. Cell Differentiation and Mineralization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Attar, H.; Kent, D. Titanium Alloys for Biomedical Implants and Devices; MDPI: Basel, Switzerland, 2021; ISBN 978-3-0365-0003-4. [Google Scholar]
- Jung, H.-D. Titanium and Its Alloys for Biomedical Applications. Metals 2021, 11, 1945. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Popov, V.V.; Muller-Kamskii, G.; Kovalevsky, A.; Dzhenzhera, G.; Strokin, E.; Kolomiets, A.; Ramon, J. Design and 3D-printing of titanium bone implants: Brief review of approach and clinical cases. Biomed. Eng. Lett. 2018, 8, 337–344. [Google Scholar] [CrossRef]
- Yuan, L.; Ding, S.; Wen, C. Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review. Bioact. Mater. 2019, 4, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Ødegaard, K.S.; Torgersen, J.; Elverum, C.W. Structural and Biomedical Properties of Common Additively Manufactured Biomaterials: A Concise Review. Metals 2020, 10, 1677. [Google Scholar] [CrossRef]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef]
- Herranz-Diez, C.; Li, Q.; Lamprecht, C.; Mas-Moruno, C.; Neubauer, S.; Kessler, H.; Manero, J.M.; Guillem-Martí, J.; Selhuber-Unkel, C. Bioactive compounds immobilized on Ti and TiNbHf: AFM-based investigations of biofunctionalization efficiency and cell adhesion. Colloids Surf. B Biointerfaces 2015, 136, 704–711. [Google Scholar] [CrossRef] [Green Version]
- Custódio, C.A.; Reis, R.L.; Mano, J.F. Engineering Biomolecular Microenvironments for Cell Instructive Biomaterials. Adv. Healthc. Mater. 2014, 3, 797–810. [Google Scholar] [CrossRef] [Green Version]
- Marín-Pareja, N.; Salvagni, E.; Guillem-Marti, J.; Aparicio, C.; Ginebra, M.P. Collagen-functionalised titanium surfaces for biological sealing of dental implants: Effect of immobilisation process on fibroblasts response. Colloids Surf. B Biointerfaces 2014, 122, 601–610. [Google Scholar] [CrossRef]
- Rao, S.; Hashemi Astaneh, S.; Villanueva, J.; Silva, F.; Takoudis, C.; Bijukumar, D.; Souza, J.C.M.; Mathew, M.T. Physicochemical and in-vitro biological analysis of bio-functionalized titanium samples in a protein-rich medium. J. Mech. Behav. Biomed. Mater. 2019, 96, 152–164. [Google Scholar] [CrossRef]
- Im, B.-J.; Lee, S.C.; Lee, M.-H.; Leesungbok, R.; Ahn, S.-J.; Kang, Y.-G.; Lee, D.Y.; Yoon, J.-H.; Lee, S.W. Promotion of osteoblastic differentiation and osteogenic transcription factor expression on a microgroove titanium surface with immobilized fibronectin or bone sialoprotein II. Biomed. Mater. 2016, 11, 035020. [Google Scholar] [CrossRef] [PubMed]
- Speziale, P.; Visai, L.; Rindi, S.; Di Poto, A. Purification of human plasma fibronectin using immobilized gelatin and Arg affinity chromatography. Nat. Protoc. 2008, 3, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Guillem-Marti, J.; Gelabert, M.; Heras-Parets, A.; Pegueroles, M.; Ginebra, M.-P.; Manero, J.M. RGD Mutation of the Heparin Binding II Fragment of Fibronectin for Guiding Mesenchymal Stem Cell Behavior on Titanium Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 3666–3678. [Google Scholar] [CrossRef] [Green Version]
- Herranz-Diez, C.; Mas-Moruno, C.; Neubauer, S.; Kessler, H.; Gil, F.J.; Pegueroles, M.; Manero, J.M.; Guillem-Marti, J. Tuning Mesenchymal Stem Cell Response onto Titanium–Niobium–Hafnium Alloy by Recombinant Fibronectin Fragments. ACS Appl. Mater. Interfaces 2016, 8, 2517–2525. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Hernandez, M.; Rappe, K.S.; Molmeneu, M.; Mas-Moruno, C.; Guillem-Marti, J.; Punset, M.; Caparros, C.; Calero, J.; Franch, J.; Fernandez-Fairen, M.; et al. Two different strategies to enhance osseointegration in porous titanium: Inorganic thermo-chemical treatment versus organic coating by peptide adsorption. Int. J. Mol. Sci. 2018, 19, 2574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girotti, A.; Orbanic, D.; Ibáñez-Fonseca, A.; Gonzalez-Obeso, C.; Rodríguez-Cabello, J.C. Recombinant Technology in the Development of Materials and Systems for Soft-Tissue Repair. Adv. Healthc. Mater. 2015, 4, 2423–2455. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Cabello, J.C.; Gonzalez De Torre, I.; González-Pérez, M.; González-Pérez, F.; Montequi, I. Fibrous Scaffolds From Elastin-Based Materials. Front. Bioeng. Biotechnol. 2021, 9, 621. [Google Scholar] [CrossRef]
- Hasan, A.; Bagnol, R.; Owen, R.; Latif, A.; Rostam, H.M.; Elsharkawy, S.; Rose, F.R.A.J.; Rodríguez-Cabello, J.C.; Ghaemmaghami, A.M.; Eglin, D.; et al. Mineralizing Coating on 3D Printed Scaffolds for the Promotion of Osseointegration. Front. Bioeng. Biotechnol. 2022, 10, 810. [Google Scholar] [CrossRef]
- Shang, Y.; Yan, Y.; Hou, X. Stimuli responsive elastin-like polypeptides and applications in medicine and biotechnology. J. Biomater. Sci. Polym. Ed. 2014, 25, 101–120. [Google Scholar] [CrossRef]
- Cipriani, F.; Bernhagen, D.; García-Arévalo, C.; de Torre, I.G.; Timmerman, P.; Rodríguez-Cabello, J.C. Bicyclic RGD peptides with high integrin α v β 3 and α 5 β 1 affinity promote cell adhesion on elastin-like recombinamers. Biomed. Mater. 2019, 14, 035009. [Google Scholar] [CrossRef]
- Flora, T.; de Torre, I.G.; Quintanilla, L.; Alonso, M.; Rodríguez-Cabello, J.C. Spatial control and cell adhesion selectivity on model gold surfaces grafted with elastin-like recombinamers. Eur. Polym. J. 2018, 106, 19–29. [Google Scholar] [CrossRef]
- Girotti, A.; Gonzalez-Valdivieso, J.; Santos, M.; Martin, L.; Arias, F.J. Functional characterization of an enzymatically degradable multi-bioactive elastin-like recombinamer. Int. J. Biol. Macromol. 2020, 164, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Misbah, M.H.; Espanol, M.; Quintanilla, L.; Ginebra, M.P.; Rodríguez-Cabello, J.C. Formation of calcium phosphate nanostructures under the influence of self-assembling hybrid elastin-like-statherin recombinamers. RSC Adv. 2016, 6, 31225–31234. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Hasan, A.; Elsharkawy, S.; Tejeda-Montes, E.; Tarakina, N.V.; Greco, G.; Nikulina, E.; Stormonth-Darling, J.M.; Convery, N.; Rodriguez-Cabello, J.C.; et al. Topographically guided hierarchical mineralization. Mater. Today Bio 2021, 11, 100119. [Google Scholar] [CrossRef] [PubMed]
- Ndao, M.; Ash, J.T.; Stayton, P.S.; Drobny, G.P. The role of basic amino acids in the molecular recognition of hydroxyapatite by statherin using solid state NMR. Surf. Sci. 2010, 604, L39–L42. [Google Scholar] [CrossRef] [Green Version]
- Vidal, E.; Guillem-Marti, J.; Ginebra, M.-P.; Combes, C.; Rupérez, E.; Rodriguez, D. Multifunctional homogeneous calcium phosphate coatings: Toward antibacterial and cell adhesive titanium scaffolds. Surf. Coat. Technol. 2020, 405, 126557. [Google Scholar] [CrossRef]
- Frosch, K.H.; Barvencik, F.; Lohmann, C.H.; Viereck, V.; Siggelkow, H.; Breme, J.; Dresing, K.; Stürmer, K.M. Migration, matrix production and lamellar bone formation of human osteoblast-like cells in porous titanium implants. Cells Tissues Organs 2002, 170, 214–227. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, Q.; Wang, J.; Li, D.; Song, Z.; Yu, J. Promotion of Osseointegration between Implant and Bone Interface by Titanium Alloy Porous Scaffolds Prepared by 3D Printing. ACS Biomater. Sci. Eng. 2020, 6, 5181–5190. [Google Scholar] [CrossRef]
- Misbah, M.H.; Santos, M.; Quintanilla, L.; Günter, C.; Alonso, M.; Taubert, A.; Rodríguez-Cabello, J.C. Recombinant DNA technology and click chemistry: A powerful combination for generating a hybrid elastin-like-statherin hydrogel to control calcium phosphate mineralization. Beilstein J. Nanotechnol. 2017, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- González, P.; González-Fernández, C.; Maqueda, A.; Pérez, V.; Escalera-Anzola, S.; de Lope, Á.R.; Arias, F.J.; Girotti, A.; Rodríguez, F.J. Silk-Elastin-like Polymers for Acute Intraparenchymal Treatment of the Traumatically Injured Spinal Cord: A First Systematic Experimental Approach. Pharmaceutics 2022, 14, 2713. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, J.C.; Girotti, A.; Ribeiro, A.; Arias, F.J. Synthesis of genetically engineered protein polymers (recombinamers) as an example of advanced self-assembled smart materials. Methods Mol. Biol. 2012, 811, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; García, A.; Girotti, A.; Alonso, M.; Rodríguez-Cabello, J.C.; González-Vázquez, A.; Planell, J.A.; Engel, E.; Buján, J.; García-Honduvilla, N.; et al. 3D silicon doped hydroxyapatite scaffolds decorated with Elastin-like Recombinamers for bone regenerative medicine. Acta Biomater. 2016, 45, 349–356. [Google Scholar] [CrossRef]
- McPherson, D.T.; Xu, J.; Urry, D.W. Product purification by reversible phase transition following Escherichia coli expression of genes encoding up to 251 repeats of the elastomeric pentapeptide GVGVP. Protein Expr. Purif. 1996, 7, 51–57. [Google Scholar] [CrossRef]
- Meyer, D.E.; Chilkoti, A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: Examples from the the elastin-like polypeptide system. Biomacromolecules 2002, 3, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Kellenbach, E.; Sanders, K.; Overbeeke, P.L.A. The Use of Proton NMR as an Alternative for the Amino Acid Analysis as Identity Test for Peptides. In NMR Spectroscopy in Pharmaceutical Analysis; Elsevier: Amsterdam, The Netherlands, 2008; pp. 429–436. ISBN 9780444531735. [Google Scholar]
- Vidal, E.; Torres, D.; Guillem-Marti, J.; Scionti, G.; Manero, J.M.; Ginebra, M.-P.; Rodríguez, D.; Rupérez, E. Titanium Scaffolds by Direct Ink Writing: Fabrication and Functionalization to Guide Osteoblast Behavior. Metals 2020, 10, 1156. [Google Scholar] [CrossRef]
- Arnaout, M.A.; Mahalingam, B.; Xiong, J.-P. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 2005, 21, 381–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejeda-Montes, E.; Smith, K.H.; Poch, M.; López-Bosque, M.J.; Martín, L.; Alonso, M.; Engel, E.; Mata, A. Engineering membrane scaffolds with both physical and biomolecular signaling. Acta Biomater. 2012, 8, 998–1009. [Google Scholar] [CrossRef]
- Tejeda-Montes, E.; Smith, K.H.; Rebollo, E.; Gómez, R.; Alonso, M.; Rodriguez-Cabello, J.C.; Engel, E.; Mata, A. Bioactive membranes for bone regeneration applications: Effect of physical and biomolecular signals on mesenchymal stem cell behavior. Acta Biomater. 2014, 10, 134–141. [Google Scholar] [CrossRef] [PubMed]
- García-Arévalo, C.; Pierna, M.; Girotti, A.; Arias, F.J.; Rodríguez-Cabello, J.C. A comparative study of cell behavior on different energetic and bioactive polymeric surfaces made from elastin-like recombinamers. Soft Matter 2012, 8, 3239. [Google Scholar] [CrossRef]
- Gobin, A.S.; West, J.L. Val-ala-pro-gly, an elastin-derived non-integrin ligand: Smooth muscle cell adhesion and specificity. J. Biomed. Mater. Res. 2003, 67A, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Punet, X.; Mauchauffé, R.; Giannotti, M.I.; Rodríguez-Cabello, J.C.; Sanz, F.; Engel, E.; Mateos-Timoneda, M.A.; Planell, J.A. Enhanced cell-material interactions through the biofunctionalization of polymeric surfaces with engineered peptides. Biomacromolecules 2013, 14, 2690–2702. [Google Scholar] [CrossRef] [PubMed]
- Raphel, J.; Parisi-Amon, A.; Heilshorn, S.C. Photoreactive elastin-like proteins for use as versatile bioactive materials and surface coatings. J. Mater. Chem. 2012, 22, 19429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierna, M.; Santos, M.; Arias, F.J.; Alonso, M.; Rodríguez-Cabello, J.C. Efficient cell and cell-sheet harvesting based on smart surfaces coated with a multifunctional and self-organizing elastin-like recombinamer. Biomacromolecules 2013, 14, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Markhoff, J.; Wieding, J.; Weissmann, V.; Pasold, J.; Jonitz-Heincke, A.; Bader, R. Influence of different three-dimensional open porous titanium scaffold designs on human osteoblasts behavior in static and dynamic cell investigations. Materials 2015, 8, 5490–5507. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.; Humayun, A.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Additively manufactured 3D porous Ti-6Al-4V constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication 2014, 6, 045007. [Google Scholar] [CrossRef]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [Green Version]
- Ruijtenberg, S.; van den Heuvel, S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.; Giachelli, C.M.; Stayton, P.S. Biomimetic peptides that engage specific integrin-dependent signaling pathways and bind to calcium phosphate surfaces. J. Biomed. Mater. Res. 2003, 67A, 69–77. [Google Scholar] [CrossRef]
- Man, K.; Brunet, M.Y.; Louth, S.; Robinson, T.E.; Fernandez-Rhodes, M.; Williams, S.; Federici, A.S.; Davies, O.G.; Hoey, D.A.; Cox, S.C. Development of a Bone-Mimetic 3D Printed Ti6Al4V Scaffold to Enhance Osteoblast-Derived Extracellular Vesicles’ Therapeutic Efficacy for Bone Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 757220. [Google Scholar] [CrossRef]









| ELR | Amino Acid Sequence | MW Da |
|---|---|---|
| IK | MESLLP[(VPGIG)2(VPGKG)(VPGIG)2]24V | 51,980 |
| H3 | MESLLP{[(VPGIG)2(VPGKG)(VPGIG)2]2 DDDEEKFLRRIGRFG [(VPGIG)2(VPGKG)(VPGIG)2]2}3V | 31,877 |
| HRGD | MGSSHHHHHHSSGLVPRGSHMESLLP{[(VPGIG)2(VPGKG)(VPGIG)2]2 AVTGRGDSPASS[(VPGIG)2(VPGKG)(VPGIG)2]2}6V | 60,661 |
| H4R4 | MESLLP{[(VPGIG)2(VPGKG)(VPGIG)2]2 DDDEEKFLRRIGRFG [(VPGIG)2(VPGKG)(VPGIG)2]2]4[[(VPGIG)2(VPGKG)(VPGIG)2] 2AVTGRGDSPASS[(VPGIG)2(VPGKG)(VPGIG)2]2]4}V | 80,730 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillem-Marti, J.; Vidal, E.; Girotti, A.; Heras-Parets, A.; Torres, D.; Arias, F.J.; Ginebra, M.-P.; Rodriguez-Cabello, J.C.; Manero, J.M. Functionalization of 3D-Printed Titanium Scaffolds with Elastin-like Recombinamers to Improve Cell Colonization and Osteoinduction. Pharmaceutics 2023, 15, 872. https://doi.org/10.3390/pharmaceutics15030872
Guillem-Marti J, Vidal E, Girotti A, Heras-Parets A, Torres D, Arias FJ, Ginebra M-P, Rodriguez-Cabello JC, Manero JM. Functionalization of 3D-Printed Titanium Scaffolds with Elastin-like Recombinamers to Improve Cell Colonization and Osteoinduction. Pharmaceutics. 2023; 15(3):872. https://doi.org/10.3390/pharmaceutics15030872
Chicago/Turabian StyleGuillem-Marti, Jordi, Elia Vidal, Alessandra Girotti, Aina Heras-Parets, Diego Torres, Francisco Javier Arias, Maria-Pau Ginebra, Jose Carlos Rodriguez-Cabello, and Jose Maria Manero. 2023. "Functionalization of 3D-Printed Titanium Scaffolds with Elastin-like Recombinamers to Improve Cell Colonization and Osteoinduction" Pharmaceutics 15, no. 3: 872. https://doi.org/10.3390/pharmaceutics15030872
APA StyleGuillem-Marti, J., Vidal, E., Girotti, A., Heras-Parets, A., Torres, D., Arias, F. J., Ginebra, M. -P., Rodriguez-Cabello, J. C., & Manero, J. M. (2023). Functionalization of 3D-Printed Titanium Scaffolds with Elastin-like Recombinamers to Improve Cell Colonization and Osteoinduction. Pharmaceutics, 15(3), 872. https://doi.org/10.3390/pharmaceutics15030872