Angiotensin II Receptor Blockers Reduce Tau/Aß42 Ratio: A Cerebrospinal Fluid Biomarkers’ Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Data Source and Variables
2.3. Statistical Analysis
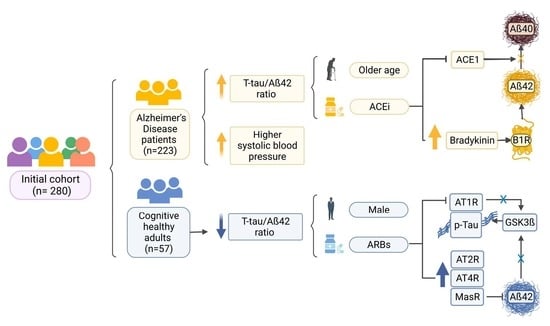
3. Results
3.1. Participants
3.2. Demographic and Clinical Data of Participants
3.3. Hypertension and Alzheimer’s Disease
3.4. Antihypertensive Drugs and Alzheimer’s Disease Biomarkers
3.5. ACEi and ARBs Pharmacological Subgroups
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Affleck, A.J.; Sachdev, P.S.; Stevens, J.; Halliday, G.M. Antihypertensive medications ameliorate Alzheimer’s disease pathology by slowing its propagation. Alzheimer’s Dement. 2020, 6, e12060. [Google Scholar] [CrossRef]
- Ribeiro, V.T.; de Souza, L.C.; Simões e Silva, A.C. Renin-Angiotensin System and Alzheimer’s Disease Pathophysiology: From the Potential Interactions to Therapeutic Perspectives. Protein Pept. Lett. 2020, 27, 484–511. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, I.; Diez-Fairen, M.; Aguilar, M.; González, J.M.; Ysamat, M.; Tartari, J.P.; Carcel, M.; Alonso, A.; Brix, B.; Arendt, P.; et al. Added value of cerebrospinal fluid multimarker analysis in diagnosis and progression of dementia. Eur. J. Neurol. 2021, 28, 1142–1152. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Roca, M.; López-Cuevas, R.; Baquero, M.; Vento, M.; Chafer-Pericas, C. Metabolomics study to identify plasma biomarkers in alzheimer disease: ApoE genotype effect. J. Pharm. Biomed. Anal. 2020, 180, 113088. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Kabir, M.T.; Rahman, M.S.; Behl, T.; Jeandet, P.; Ashraf, G.M.; Najda, A.; Bin-Jumah, M.N.; El-Seedi, H.R.; Abdel-Daim, M.M. Revisiting the Amyloid Cascade Hypothesis: From Anti-Aβ Therapeutics to Auspicious New Ways for Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 5858. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, N.Q.; Yan, F.; Jin, H.; Zhou, S.Y.; Shi, J.S.; Jin, F. Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav. Brain Res. 2018, 339, 57–65. [Google Scholar] [CrossRef]
- Hestad, K.A.; Horndalsveen, P.O.; Engedal, K. Blood Pressure and T-Tau in Spinal Fluid Are Associated With Delayed Recall in Participants With Memory Complaints and Dementia of the Alzheimer’s Type. Front. Aging Neurosci. 2021, 13, 685. [Google Scholar] [CrossRef]
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Münzel, T. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int. J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef]
- Ramos, H.; Moreno, L.; Gil, M.; García-Lluch, G.; Sendra-Lillo, J.; Alacreu, M. Pharmacists’ Knowledge of Factors Associated with Dementia: The A-to-Z Dementia Knowledge List. Int. J. Environ. Res. Public Health 2021, 18, 9934. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. Envejecimiento y salud. Cent Prensa OMS. 2018, pp. 1–2. Available online: https://www.who.int/es/news-room/fact-sheets/detail/envejecimiento-y-salud (accessed on 5 May 2020).
- Wahidi, N.; Lerner, A.J. Blood Pressure Control and Protection of the Aging Brain. Neurotherapeutics 2019, 16, 569–579. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Wharton, W.; Zhao, L.; Steenland, K.; Goldstein, F.C.; Schneider, J.A.; Barnes, L.L.; Gearing, M.; Yasar, S. Neurofibrillary Tangles and Conversion to Mild Cognitive Impairment with Certain Antihypertensives. J. Alzheimers Dis. 2019, 70, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Lebouvier, T.; Chen, Y.; Duriez, P.; Pasquier, F.; Bordet, R. Antihypertensive agents in Alzheimer’s disease: Beyond vascular protection. Expert Rev. Neurother. 2020, 20, 175–187. [Google Scholar] [CrossRef] [Green Version]
- den Brok, M.G.; van Dalen, J.W.; Abdulrahman, H.; Larson, E.B.; van Middelaar, T.; van Gool, W.A.; van Charante, E.P.; Richard, E. Antihypertensive Medication Classes and the Risk of Dementia: A Systematic Review and Network Meta-Analysis. J. Am. Med. Dir. Assoc. 2021, 22, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Carretero, M. Doxazosina|Offarm. Doxazosina. 2002, pp. 176–178. Available online: https://www.elsevier.es/es-revista-offarm-4-articulo-doxazosina-13035880 (accessed on 24 April 2022).
- Coelho, B.P.; Gaelzer, M.M.; dos Santos Petry, F.; Hoppe, J.B.; Trindade, V.M.; Salbego, C.G.; Guma, F.T. Dual Effect of Doxazosin: Anticancer Activity on SH-SY5Y Neuroblastoma Cells and Neuroprotection on an In Vitro Model of Alzheimer’s Disease. Neuroscience 2019, 404, 314–325. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Xu, Y.-F.; Liu, Y.-H.; Yin, J.; Wang, J.-Z. Nitric oxide induces tau hyperphosphorylation via glycogen synthase kinase-3b activation. FEBS Lett. 2005, 579, 6230–6236. [Google Scholar] [CrossRef] [Green Version]
- Sawmiller, D.; Habib, A.; Li, S.; Darlington, D.; Hou, H.; Tian, J.; Shytle, R.D.; Smith, A.; Giunta, B.; Mori, T.; et al. Diosmin reduces cerebral Aβ levels, tau hyperphosphorylation, neuroinflammation, and cognitive impairment in the 3xTg-AD Mice. J. Neuroimmunol. 2016, 299, 98. [Google Scholar] [CrossRef] [Green Version]
- Bachmeier, C.; Beaulieu-Abdelahad, D.; Mullan, M.; Paris, D. Selective dihydropyiridine compounds facilitate the clearance of β-amyloid across the blood-brain barrier. Eur. J. Pharmacol. 2011, 659, 124–129. [Google Scholar] [CrossRef]
- Chuang, Y.F.; Breitner, J.C.; Chiu, Y.L.; Khachaturian, A.; Hayden, K.; Corcoran, C.; Tschanz, J.; Norton, M.; Munger, R.; Welsh-Bohmer, K.; et al. Use of diuretics is associated with reduced risk of Alzheimer’s disease: The Cache County Study. Neurobiol. Aging 2014, 35, 2429–2435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, D.; Kim, S.; Choi, H.; Oh, I.H.; Kim, B.S.; Choi, H.R.; Kim, S.Y.; Won, C.W. Calcium-Channel Blockers and Dementia Risk in Older Adults—National Health Insurance Service—Senior Cohort (2002–2013). Circ. J. 2016, 80, 2336–2342. [Google Scholar] [CrossRef] [Green Version]
- Barus, R.; Béné, J.; Deguil, J.; Gautier, S.; Bordet, R. Drug interactions with dementia-related pathophysiological pathways worsen or prevent dementia. Br. J. Pharmacol. 2019, 176, 3413–3434. [Google Scholar] [CrossRef]
- Scotti, L.; Bassi, L.; Soranna, D.; Verde, F.; Silani, V.; Torsello, A.; Parati, G.; Zambon, A. Association between renin-angiotensin-aldosterone system inhibitors and risk of dementia: A meta-analysis. Pharmacol. Res. 2021, 166, 105515. [Google Scholar] [CrossRef] [PubMed]
- D’Silva, E.; Meor Azlan, N.F.; Zhang, J. Angiotensin II Receptor Blockers in the Management of Hypertension in Preventing Cognitive Impairment and Dementia—A Systematic Review. Pharmaceutics 2022, 14, 2123. [Google Scholar] [CrossRef]
- Evans, C.E.; Miners, J.S.; Piva, G.; Willis, C.L.; Heard, D.M.; Kidd, E.J.; Good, M.A.; Kehoe, P.G. ACE2 activation protects against cognitive decline and reduces amyloid pathology in the Tg2576 mouse model of Alzheimer’s disease. Acta Neuropathol. 2020, 139, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouk, M.; Wu, C.Y.; Rabin, J.S.; Edwards, J.D.; Ramirez, J.; Masellis, M.; Swartz, R.H.; Herrmann, N.; Lanctôt, K.L.; Black, S.E.; et al. Associations between brain amyloid accumulation and the use of angiotensin-converting enzyme inhibitors versus angiotensin receptor blockers. Neurobiol. Aging 2021, 100, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, F.; Camins, A.; Ettcheto, M.; Bicker, J.; Falcão, A.; Cruz, M.T.; Fortuna, A. Targeting brain Renin-Angiotensin System for the prevention and treatment of Alzheimer’s disease: Past, present and future. Ageing Res. Rev. 2022, 77, 101612. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Hughes, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Pfeffer, R.I.; Kurosaki, T.T.; Harrah, C.H.; Chance, J.M.; Filos, S. Measurement of functional activities in older adults in the community. J. Gerontol. 1982, 37, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Galasko, D.; Bennett, D.; Sano, M.; Ernesto, C.; Thomas, R.; Grundman, M.; Ferris, S. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. Alzheimer Dis. Assoc. Disord. 1997, 11, 33–39. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; López-Cuevas, R.; Cuevas, A.; Baquero, M.; Cháfer-Pericás, C. Lipid peroxidation biomarkers correlation with medial temporal atrophy in early Alzheimer Disease. Neurochem. Int. 2019, 129, 104519. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Álvarez-Sánchez, L.; Ferrer, I.; López-Nogueroles, M.; Cañada-Martínez, A.J.; Oger, C.; Galano, J.M.; Durand, T.; Baquero, M.; Cháfer-Pericás, C. Lipid Peroxidation Assessment in Preclinical Alzheimer Disease Diagnosis. Antioxidants 2021, 10, 1043. [Google Scholar] [CrossRef]
- Guo, Z.; Fratiglioni, L.; Zhu, L.; Fastbom, J.; Winblad, B.; Viitanen, M. Occurrence and Progression of Dementia in a Community Population Aged 75 Years and Older Relationship of Antihypertensive Medication Use. Arch. Neurol. 1999, 56, 991–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, M.D.; Lane, K.A.; Gao, S.; Evans, R.M.; Unverzagt, F.W.; Hall, K.S.; Hendrie, H. Preservation of cognitive function with antihypertensive medications: A longitudinal analysis of a community-based sample of African Americans. Arch. Intern. Med. 2002, 162, 2090–2096. [Google Scholar] [CrossRef] [Green Version]
- Wharton, W.; Stein, J.H.; Korcarz, C.; Sachs, J.; Olson, S.R.; Zetterberg, H.; Dowling, M.; Ye, S.; Gleason, C.E.; Underbakke, G.; et al. The Effects of Ramipril in Individuals at Risk for Alzheimer’s Disease: Results of a Pilot Clinical Trial NIH Public Access. J. Alzheimers. Dis. 2012, 32, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nation, D.A.; Ho, J.; Yew, B. Older Adults Taking AT1-Receptor Blockers Exhibit Reduced Cerebral Amyloid Retention. J. Alzheimer’s Dis. 2016, 50, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Angulo, J.; Cuevas, P.; Fernández, A.; Gabancho, S.; Videla, S.; Sáenz De Tejada, I. Calcium dobesilate potentiates endothelium-derived hyperpolarizing factor-mediated relaxation of human penile resistance arteries. Br. J. Pharmacol. 2003, 139, 854. [Google Scholar] [CrossRef]
- Ruiz, E.; Lorente, R.; Tejerina, T. Effects of calcium dobesilate on the synthesis of endothelium-dependent relaxing factors in rabbit isolated aorta. Br. J. Pharmacol. 1997, 121, 711–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siciliano, R.; Barone, E.; Calabrese, V.; Rispoli, V.; Allan Butterfield, D.; Mancuso, C. Experimental research on nitric oxide and the therapy of Alzheimer disease: A challenging bridge. CNS Neurol. Disord. Drug Targets 2011, 10, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Kumar Nath, A.; Ghosh Dey, S. Dalton Transactions FRONTIER Simultaneous binding of heme and Cu with amyloid β peptides: Active site and reactivities. Dalton Trans. 2022, 51, 4986–4999. [Google Scholar] [CrossRef] [PubMed]
- Sadleir Id, K.R.; Popovic, J.; Khatri, A.; Vassar, R. Oral nimodipine treatment has no effect on amyloid pathology or neuritic dystrophy in the 5XFAD mouse model of amyloidosis. PLoS ONE 2022, 17, e0263332. [Google Scholar] [CrossRef]
- Yasar, S.; Corrada, M.; Brookmeyer, R.; Kawas, C. Calcium channel blockers and risk of AD: The Baltimore Longitudinal Study of Aging. Neurobiol. Aging 2005, 26, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Yasar, S.; Xia, J.; Yao, W.; Furberg, C.D.; Xue, Q.L.; Mercado, C.I.; Fitzpatrick, A.L.; Fried, L.P.; Kawas, C.H.; Sink, K.M.; et al. Antihypertensive drugs decrease risk of Alzheimer disease. Neurology 2013, 81, 896–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawlor, B.; Segurado, R.; Kennelly, S.; Olde Rikkert, M.G.; Howard, R.; Pasquier, F.; Börjesson-Hanson, A.; Tsolaki, M.; Lucca, U.; Molloy, D.W.; et al. Nilvadipine in mild to moderate Alzheimer disease: A randomised controlled trial. Fani Tsolaki-TagarakiID 2018, 25, 39. [Google Scholar] [CrossRef]
- Viel, A.T.; Buck, S.H. Kallikrein-kinin system mediated inflammation in Alzheimer’s disease in vivo. Curr. Alzheimer Res. 2011, 8, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Asraf, K.; Torika, N.; Danon, A.; Fleisher-Berkovich, S. Involvement of the bradykinin B1 Receptor in microglial activation: In vitro and in vivo studies. Front. Endocrinol. (Lausanne) 2017, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.L.; Singh, P.; Wong, J.; Horn, K.; Strickland, S.; Norris, E.H. An antibody against HK blocks Alzheimer’s disease peptide β-amyloid-induced bradykinin release in human plasma. Proc. Natl. Acad. Sci. USA 2019, 116, 22921–22923. [Google Scholar] [CrossRef] [Green Version]
- Dutra, R.C. Kinin receptors: Key regulators of autoimmunity. Autoimmun. Rev. 2017, 16, 192–207. [Google Scholar] [CrossRef]
- Vivek Kumar, S.; Thakur Gurjeet, S. Navigating Alzheimer’s Disease vía Chronic Stress: The Role of Glucocorticoids. Curr. Drug Targets 2020, 21, 433–444. [Google Scholar] [CrossRef]
- Gebre, A.K.; Altaye, B.M.; Atey, T.M.; Tuem, K.B.; Berhe, D.F. Targeting Renin-Angiotensin System Against Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 440. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Chen, Z.L.; Strickland, S.; Norris, E.H. Increased Contact System Activation in Mild Cognitive Impairment Patients with Impaired Short-Term Memory. J. Alzheimers Dis. 2020, 77, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, R.M.; de Souza, A.C.; Bicca, M.A.; Pamplona, F.A.; de Mello, N.; Passos, G.F.; Medeiros, R.; Takahashi, R.N.; Calixto, J.B.; Prediger, R.D. Blockade of hippocampal bradykinin B1 receptors improves spatial learning and memory deficits in middle-aged rats. Behav. Brain Res. 2017, 316, 74–81. [Google Scholar] [CrossRef]
- Mugisho, O.O.; Robilliard, L.D.; Nicholson, L.F.B.; Graham, E.S.; O’Carroll, S.J. Bradykinin receptor-1 activation induces inflammation and increases the permeability of human brain microvascular endothelial cells. Cell Biol. Int. 2019, 44, 343–351. [Google Scholar] [CrossRef]
- Zhong, K.L.; Chen, F.; Hong, H.; Ke, X.; Lv, Y.G.; Tang, S.S.; Zhu, Y.B. New views and possibilities of antidiabetic drugs in treating and/or preventing mild cognitive impairment and Alzheimer’s Disease. Metab. Brain Dis. 2018, 33, 1009–1018. [Google Scholar] [CrossRef]
- Ji, B.; Wang, Q.; Xue, Q.; Li, W.; Li, X.; Wu, Y. The Dual Role of Kinin/Kinin Receptors System in Alzheimer’s Disease. Front. Mol. Neurosci. 2019, 12, 234. [Google Scholar] [CrossRef]
- Vipin, A.; Ng, K.K.; Ji, F.; Shim, H.Y.; Lim, J.K.; Pasternak, O.; Zhou, J.H.; Alzheimer’s Disease Neuroimaging Initiative. Amyloid burden accelerates white matter degradation in cognitively normal elderly individuals. Hum. Brain Mapp. 2019, 40, 2065. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yu, R.; Wang, H.; Zheng, R. Effects of rivastigmine hydrogen tartrate and donepezil hydrochloride on the cognitive function and mental behavior of patients with Alzheimer’s disease. Exp. Ther. Med. 2020, 20, 1789–1795. [Google Scholar] [CrossRef]
- Urmila, A.; Rashmi, P.; Nilam, G.; Subhash, B. Recent Advances in the Endogenous Brain Renin-Angiotensin System and Drugs Acting on It. J. Renin Angiotensin Aldosterone Syst. 2021, 2021, 9293553. [Google Scholar] [CrossRef] [PubMed]
- Naffah-Mazzacoratti M da, G.; Gouveia, T.L.F.; Simões, P.S.R.; Perosa, S.R. What have we learned about the kallikrein-kinin and renin-angiotensin systems in neurological disorders? World J. Biol. Chem. 2014, 5, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, I.; Brown, L.; Mack, W.J.; Chui, H. Impact of Angiotensin Receptor Blockers on Alzheimer Disease Neuropathology in a Large Brain Autopsy Series. Arch. Neurol. 2012, 69, 1632–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Variable | AD (n = 223) | Control (n = 57) |
|---|---|---|
| Age (years, median (IQR)) | 71 (67.5, 74) | 65 (62, 69) |
| Sex (female, n (%)) | 135 (60.54%) | 25 (43.86%) |
| CSF biomarkers | ||
| Aß42 levels (pg/mL, median (IQR)) | 600 (468.04, 702.1) | 1206.15 (996, 1472) |
| t-tau levels (pg/mL, median (IQR)) | 586 (414, 837) | 240 (182, 313) |
| p-tau levels (pg/mL, median (IQR)) | 92 (72.5, 131) | 42 (32, 56) |
| Ratio t-tau/Aß42 (median (IQR)) | 0.94 (0.68, 1.43) | 0.19 (0.14, 0.24) |
| Smoking history | ||
| No (n, %) | 145 (65.02%) | 34 (59.65%) |
| No (Ex-smoker), (n, %) | 45 (20.18%) | 10 (17.54%) |
| Yes (n, %) | 33 (14.8%) | 13 (22.81%) |
| Number of concomitant medications | 5 (3, 7) | 3 (2, 5) |
| Total cholesterol (mg/dL, median (IQR)) | 189 (165.25, 212) | 196 (170, 222) |
| HDL cholesterol (mg/dL, median (IQR)) | 55.5 (47, 66.25) | 55 (44, 67.6) |
| Lipid-modifying agents (n, %) | 123 (55.16%) | 28 (49.12%) |
| Antidiabetic drugs (n, %) | 36 (16.14%) | 11 (19.3%) |
| Systolic blood pressure (mmHg, median (IQR)) | 135 (124.58, 143.42) | 130 (118, 139) |
| Diastolic blood pressure (DBP) (mmHg, median (IQR)) | 75 (70, 81) | 78 (71, 82.5) |
| Hypertension (n, %) | 122 (54.71%) | 30 (52.63%) |
| Variable | AD (n = 223) | Control (n = 57) |
|---|---|---|
| Antihypertensive drugs prescription (n, %) | 116 (52.02%) | 29 (50.88%) |
| Age at first prescribed antihypertensive treatment (years, median (IQR)) | 61 (58, 65) | 56 (53, 62) |
| Years since 1st antihypertensive treatment prescription (years, median (IQR)) | 7 (0, 11) | 2 (0, 10) |
| Number of antihypertensives daily intake (n, %) | ||
| 0 | 107 (47.98%) | 28 (49.12%) |
| 1 | 59 (26.46%) | 11 (19.3%) |
| 2 | 40 (17.94%) | 11 (19.3%) |
| 3 | 16 (7.17%) | 6 (10.53%) |
| 4 | 1 (0.45%) | 0 (0%) |
| 5 | 0 (0%) | 1 (1.75%) |
| Doxazosin prescription (n, %) | 2 (0.9%) | 2 (3.51%) |
| Diuretics prescription (n, %) | 9 (4.04%) | 3 (5.26%) |
| Peripheral vasodilators (n, %) | 3 (1.35%) | 1 (1.75%) |
| Calcium dobesilate prescription (n, %) | 0 (0%) | 1 (1.75%) |
| Beta-blocking agents prescription (n, %) | 20 (8.97%) | 1 (1.75%) |
| Calcium channel blockers prescription (n, %) | 36 (16.14%) | 8 (14.04%) |
| Agents acting on the renin-angiotensin system (n, %) | 90 (40.36%) | 25 (43.86%) |
| Variable | Aß42 Model (Estimate) | p-tau Model (Estimate) | t-tau Model (Estimate) | Ratio tau/Aß42 Amyloid Model (Estimate) |
|---|---|---|---|---|
| Sex (male) | - | - | −0.087 | −0.1 |
| Age | −0.015 | 0.015 | 0.024 | 0.047 |
| Antidiabetic drugs | 0.043 | - | - | −0.035 |
| Total cholesterol (mg/dL) | - | - | −0.001 | −0.001 |
| Diastolic blood pressure | - | - | −0.002 | - |
| Number of antihypertensives | 0 | - | - | - |
| Doxazosin prescription | 0.123 | - | −0.107 | −0.481 |
| Calcium dobesilate prescription | 0.175 | - | - | −0.421 |
| Calcium channel blockers | - | - | - | 0.053 |
| ACEi, plain | - | - | 0.059 | 0.05 |
| ARBs, combinations | - | - | −0.041 | −0.206 |
| lambda | 0.067 | 0.123 | 0.069 | 0.075 |
| Variable | AD | Control |
|---|---|---|
| Angiotensin-converting enzyme inhibitors prescription (n, (%)) | 40 (17.94) | 5 (8.77) |
| Angiotensin-converting enzyme inhibitors prescription duration (months, median (IQR)) | 42 (11, 106) | 19 (1, 48) |
| Plain angiotensin-converting enzyme inhibitors prescription prescription (n, (%)) | 30 (13.45) | 1 (1.75) |
| Combinations of angiotensin-converting enzyme inhibitors prescription prescription (n, (%)) | 10 (4.48%) | 4 (7.02%) |
| Angiotensin-converting enzyme inhibitors prescription without medical records of angiotensin II receptor blockers prescription (n, (%)) | 33 (14.8%) | 4 (7.02%) |
| Angiotensin II receptor blockers prescription (n, (%)) | 50 (22.42%) | 20 (35.09%) |
| Angiotensin II receptor blockers duration (months, median (IQR)) | 83 (56.75, 118.75) | 69 (39, 95) |
| Plain angiotensin II receptor blockers (n, (%)) | 21 (9.42%) | 7 (12.28%) |
| Combinations of angiotensin II receptor blockers (n, (%)) | 29 (13%) | 12 (21.05%) |
| Angiotensin II receptor blockers prescription without medical records of angiotensin-converting enzyme inhibitors prescription (n, (%)) | 43 (19.28) | 18 (31.58) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Lluch, G.; Peña-Bautista, C.; Royo, L.M.; Baquero, M.; Cañada-Martínez, A.J.; Cháfer-Pericás, C. Angiotensin II Receptor Blockers Reduce Tau/Aß42 Ratio: A Cerebrospinal Fluid Biomarkers’ Case-Control Study. Pharmaceutics 2023, 15, 924. https://doi.org/10.3390/pharmaceutics15030924
García-Lluch G, Peña-Bautista C, Royo LM, Baquero M, Cañada-Martínez AJ, Cháfer-Pericás C. Angiotensin II Receptor Blockers Reduce Tau/Aß42 Ratio: A Cerebrospinal Fluid Biomarkers’ Case-Control Study. Pharmaceutics. 2023; 15(3):924. https://doi.org/10.3390/pharmaceutics15030924
Chicago/Turabian StyleGarcía-Lluch, Gemma, Carmen Peña-Bautista, Lucrecia Moreno Royo, Miguel Baquero, Antonio José Cañada-Martínez, and Consuelo Cháfer-Pericás. 2023. "Angiotensin II Receptor Blockers Reduce Tau/Aß42 Ratio: A Cerebrospinal Fluid Biomarkers’ Case-Control Study" Pharmaceutics 15, no. 3: 924. https://doi.org/10.3390/pharmaceutics15030924
APA StyleGarcía-Lluch, G., Peña-Bautista, C., Royo, L. M., Baquero, M., Cañada-Martínez, A. J., & Cháfer-Pericás, C. (2023). Angiotensin II Receptor Blockers Reduce Tau/Aß42 Ratio: A Cerebrospinal Fluid Biomarkers’ Case-Control Study. Pharmaceutics, 15(3), 924. https://doi.org/10.3390/pharmaceutics15030924