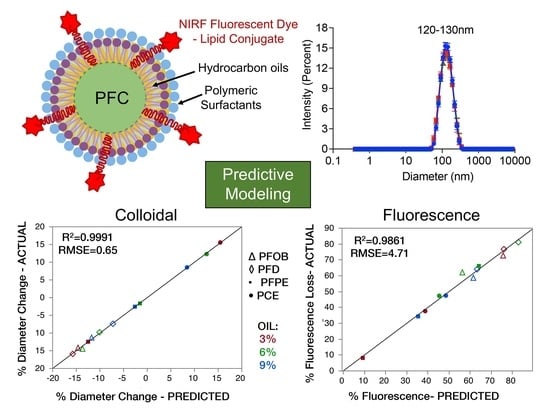
3.1. Study Design and Identification of Critical Quality Attribute and Quality Control Testing Conditions
In previous studies, our group was able to successfully model the colloidal stability of microemulsions in terms of percent diameter change after 30 days storage [
13], and nanoemulsions in terms of percent diameter change over 90 days storage [
14]. In these earlier studies, formulations were stored at ambient temperature. Thus, 30 days or longer of storage was required before accurate models could be developed to predict formulation stability. Ideally, accelerated stability testing could be employed to identify the parameters that have a significant impact on nanoemulsion colloidal stability, as this would allow for identification of suitable candidates for further testing and optimization in a time-efficient manner. Thus, in the presented work, nanoemulsions were subjected to incubation at 80 °C for 7 days as a form of accelerated stability testing (
Table 2). In a clinical setting, nanoemulsions would not be subjected to this harsh condition. For this reason, this test was defined as a quality control (QC) test.
For accelerated stability model predictions to be meaningful, they must accurately reflect the behavior of the formulations under non-accelerated (storage) conditions. To test this, nanoemulsions in the presented study were also evaluated for percent diameter change and PDI after 95 days (~3 months) and 215 days (~7 months) storage at 4 °C (
Table 2). Nanoemulsion colloidal stability upon storage is essential to final product efficacy, so percent diameter change and PDI after 95 days storage at 4 °C were defined as CQAs. Nanoemulsion stability beyond 6 months is preferable, but not required, so nanoemulsion percent diameter change and PDI after 215 days storage at 4 °C were defined as QC parameters. This extended shelf-life evaluation was included as an additional time point that could be used to evaluate the predictive relevancy of the accelerated stability models.
To be tested in vitro and in vivo, it is necessary that nanoemulsion diameter and PDI are not impacted by filtration through a 0.22 µm filter, as this is the method of sterilization for these formulations. Further, it is essential that nanoemulsions maintain colloidal stability upon exposure to conditions encountered during cell culture. Therefore, nanoemulsion percent diameter change and PDI upon filtration and exposure to cell culture conditions were defined as CQAs (
Table 2). Specifically, exposure to cell culture conditions consisted of nanoemulsion dilution at 1:80
v/
v in cell culture media and incubation of the resulting dilution at 37 °C for 3 h, followed by centrifugation at 1100 rpm for 5 min. This sequence of testing conditions was selected because it simulates conditions nanoemulsions could experience upon ex vivo cell labeling, a potential application of these products [
10]. All diameter and PDI CQA and QC testing descriptions and specifications are summarized in
Table 2.
CQAs, CQA specifications, and QC testing conditions were also identified for nanoemulsion fluorescence stability (
Table 3). Fluorescence CQAs were defined as the percent fluorescence signal loss upon (1) incubation of undiluted nanoemulsion at 25 °C for 24 h, (2) incubation of nanoemulsion diluted in cell culture media at 37 °C for 2 h, or (3) storage at 4 °C for 95 days. Incubation at 25 °C for 24 h was defined to account for times that the nanoemulsion may be left at ambient temperature during transportation or on a benchtop prior to use. Incubation in cell culture media at 37 °C for 2 h was defined to mimic conditions nanoemulsions will experience upon use in vitro in cell culture assays. The nanoemulsions are designed to passively target macrophages, which phagocytose nanoemulsion droplets within 2 h of exposure in cell culture. As forms of accelerated stability testing, percent fluorescence signal loss was also assessed after incubation of undiluted nanoemulsion at 25 °C for 72 h, and after incubation of nanoemulsion diluted in cell culture media at 37 °C for 12 h. These extended incubation times were defined as QC tests (
Table 3). Additionally, nanoemulsion percent fluorescence signal loss after 215 days storage at 4 °C was included as a QC test, so that fluorescence signal loss could be assessed in parallel with nanoemulsion percent diameter change over the shelf life of the product.
As mentioned in the introduction, the primary goals of this work were to identify stable nanoemulsions with high perfluorocarbon loading (30%
w/
v) and minimal hydrocarbon concentration, and to understand the parameters that have an impact on the stability of the fluorescent reporter in these complex nanoemulsion formulations. To achieve these goals, a full factorial design of experiments (DoE) was developed to study the impact of nanoemulsion perfluorocarbon type and hydrocarbon concentration on nanoemulsion colloidal and fluorescent stability (
Table 4). Three levels were defined for hydrocarbon concentration (3, 6, and 9%
w/
v), and four levels were defined for perfluorocarbon type (PFOB, PFD, PFPE, and PCE), resulting in a 12-run full factorial design. Nanoemulsion formulations are summarized in
Table 4, and perfluorocarbon structures are shown in
Figure 1. Nanoemulsions were developed using minimal processing (30 s sonication and four passes on the microfluidizer), as previous work [
14] demonstrated that nanoemulsion processing, particularly sonication time, can decrease nanoemulsion drug encapsulation efficiency. Thus, in this work, processing conditions were limited to maximize incorporation of the fluorescent reporter (DiD).
3.2. Assessment of Nanoemulsion Colloidal and Fluorescence Stability with Multiple Linear Regression (MLR) Modeling
All developed nanoemulsions were subjected to the colloidal and fluorescence stability CQA and QC tests summarized in
Table 2 and
Table 3. The results of the CQA testing are summarized in
Supplemental Table S1 and the results of the QC testing are summarized in
Supplemental Table S2. The majority of nanoemulsion formulations (eight of twelve) met all colloidal CQA specifications. These include baseline diameter and PDI, as well as nanoemulsion percent diameter change and PDI after filtration, after exposure to cell culture conditions, and after 95 days storage at 4 °C (
Supplemental Table S1). Three of the four nanoemulsions that failed to meet one or more colloidal CQA specifications contained 3%
w/
v olive oil, suggesting that olive oil concentrations higher than 3%
w/
v are required to achieve colloidally stable nanoemulsions with 30%
w/
v perfluorocarbon, regardless of perfluorocarbon type. An unexpected finding was that PFOB and PFD nanoemulsions at all olive oil concentrations decrease in diameter over 95 days storage at 4 °C, as shown by negative percent diameter change values. This decrease in diameter (negative percent diameter change value) is also observed for all PFOB and PFD nanoemulsions after incubation at 80 °C for 7 days, and after 215 days storage at 4 °C (
Supplemental Table S2).
Another unexpected finding was that that vast majority of nanoemulsions failed to meet one or more fluorescence CQA specification (
Supplemental Table S1). Previously developed tri-phasic, perfluorocarbon nanoemulsions containing NIRF dyes have been evaluated in a murine model of complete Freund’s adjuvant (CFA) induced inflammation [
15], a murine subcutaneous breast carcinoma model [
8], and a rat CCI model [
5]. In these studies, significant loss of fluorescence signal was not observed, and fluorescence signal could be detected in vivo 40 days after a single i.v. nanoemulsion administration [
15], suggesting superior stability of the NIRF dye in vivo. To understand the parameters driving fluorescence signal loss in the presented formulations, MLR models were developed to predict nanoemulsion baseline (day 1) fluorescence signal, nanoemulsion fluorescence signal loss after 72 h incubation at 25 °C, and nanoemulsion percent fluorescence signal loss after 12 h incubation in cell culture media at 37 °C (
Figure 2,
Table 5).
Olive oil concentration is the most significant parameter driving baseline fluorescence signal, with a model
p-value < 0.0001 (
Table 5). The actual vs. predicted plot highlights this, as three clusters of formulations grouped by oil type are observed (
Figure 2A), and the bar plot shows increasing fluorescence signal with increasing olive oil concentration across all perfluorocarbon types (
Figure 2B). Perfluorocarbon type was also found to have a significant impact on baseline fluorescence (
p-value 0.04487). The scaled coefficients for the PFD and PFOB terms are negative, indicating that nanoemulsions containing these perfluorocarbons have a decreased baseline fluorescence signal compared to nanoemulsions containing PFPE and PCE that is independent of olive oil concentration (
Table 5). With the largest positive scaled coefficient, nanoemulsions containing PFPE were determined to have the highest baseline fluorescence signal (
Table 5).
MLR models developed to predict nanoemulsion percent fluorescence signal loss after 72 h incubation at 25 °C or after 12 h incubation in cell culture media at 37 °C are comparable (
Figure 2,
Table 5). Similar conclusions can be drawn from both models, suggesting that nanoemulsions respond similarly to the two different stressors. Perfluorocarbon type was found to significantly contribute to both models, with a model
p-value of <0.00001 for the 37 °C model and a
p-value of 0.00014 for the 25 °C model. In both models, scaled coefficients indicate that fluorescence signal loss is greater in nanoemulsions containing PFOB and PFD (
Table 5). The actual vs. predicted plots highlight this, as distinct separations can be observed dividing PFOB and PFD nanoemulsions from PFPE and PCE nanoemulsions (
Figure 2C,E). Bar plots also show that fluorescence signal loss is greater in PFOB and PFD nanoemulsions (
Figure 2D,F).
Olive oil concentration was also found to significantly contribute to fluorescence signal loss, with a
p-value < 0.0001 in the 37 °C model and a
p-value of 0.0254 in the 25 °C model (
Table 5). Scaled coefficients for the olive oil concentration terms are positive in both models, indicating that increasing olive oil concentration results in a greater loss of fluorescence signal. This trend is particularly apparent in the 37 °C model (
Figure 2F). Together, the fluorescence models demonstrate that increasing olive oil concentration will increase nanoemulsion baseline fluorescence signal, but at a cost, as increasing olive oil concentration results in greater fluorescence signal loss in response to stresses (i.e., elevated temperature and dilution in cell culture media). PFPE and PCE nanoemulsions appear more suitable for the incorporation of fluorescent dyes, as nanoemulsions containing these perfluorocarbons had higher baseline fluorescence signal and lower fluorescence signal loss in response to stress when compared to nanoemulsions containing PFOB and PFD.
After investigating the parameters that influence baseline fluorescence signal and fluorescence stability, MLR models were developed to understand the impact of olive oil concentration and perfluorocarbon type on nanoemulsion PDI after exposure to stress (exposure to cell culture conditions) and after extended storage (215 days at 4 °C). These two models were developed in parallel to assess whether results of the stress stability test could be used to accurately predict nanoemulsion behavior upon long-term storage. Model results are shown in
Figure 3 and
Table 6.
The cell culture conditions test was designed to mimic the conditions that nanoemulsions could be exposed to upon ex vivo labeling conditions. This test is described in
Section 2.5.2. An accurate MLR model was developed to predict nanoemulsion PDI after exposure to cell culture conditions (
Figure 3A,B,
Table 6). Perfluorocarbon type (
p-value 0.00025) and olive oil concentration (
p-value 0.00951) both significantly contribute to nanoemulsion PDI after exposure to cell culture conditions, and a significant interaction between perfluorocarbon type and olive oil concentration was detected (
p-value 0.04763). The actual vs. predicted plot (
Figure 3A) and bar plot (
Figure 3B) show that nanoemulsions containing PFOB and PFD have higher PDI values than nanoemulsions with PFPE and PCE. Accordingly, the scaled coefficients for perfluorocarbon type are positive for PFOB and PFD and negative for PFPE and PCE (
Table 6). Olive oil concentration has a negative scaled coefficient estimate, indicating that increasing olive oil concentration reduces nanoemulsion PDI in response to cell culture conditions. This effect is most apparent for PFOB and PFD nanoemulsions (
Figure 3A,B).
The MLR model for PDI after 215 days storage at 4 °C was comparable to the model for PDI after exposure to cell culture conditions in several ways. Perfluorocarbon type (
p-value 0.00112), olive oil concentration (
p-value 0.03055) and an interaction between these two parameters (
p-value 0.02171) all contributed significantly to PDI after 215 days storage (
Table 6). Scaled coefficient values indicate that increasing olive oil concentration reduces PDI, and that nanoemulsions with PFOB and PFD have larger PDI values compared to nanoemulsions containing PFPE and PCE. These trends can be seen in the model actual vs. predicted plot (
Figure 3C) and in the bar plot of PDI values (
Figure 3D). Therefore, the MLR model for PDI after exposure to cell culture conditions was able to estimate nanoemulsion PDI trends upon long-term storage.
High PDI values indicate that a nanoemulsion has a broader droplet diameter range, which can decrease nanoemulsion colloidal stability. Thus, together these data suggest that nanoemulsions containing PFOB and PFD may be less stable compared to nanoemulsions containing PFPE and PCE. This possibility was further investigated by developing MLR models to predict nanoemulsion percent diameter change after 7 days incubation at 80 °C (
Figure 4A,B,
Table 7) and after 215 days storage at 4 °C (
Figure 4C,D,
Table 7). Similar to PDI, an accelerated stability model and a shelf-life stability model were developed in parallel to assess whether results of the accelerated stability test could be used to accurately predict nanoemulsion behavior upon long-term storage. Nanoemulsion percent diameter change after 7 days storage at 80 °C was found to be dependent on perfluorocarbon type (
p-value 0.0005) and the interaction between olive oil concentration and perfluorocarbon type (
p-value 0.0367). Interestingly, incubation at 80 °C resulted in a reduction in average nanoemulsion diameter for nanoemulsions containing PFOB and PFD (
Figure 4A,B,
Table 7). Average diameter of PFPE nanoemulsions remained relatively unaffected over the incubation period, while average diameter of PCE nanoemulsions increased. This increase was significantly dependent upon olive oil concentration, as the interaction term between olive oil concentration and PCE had a
p-value of 0.0083. This suggests that increasing olive oil concentration may improve the colloidal stability of PCE nanoemulsions.
An MLR model was next developed to predict nanoemulsion percent diameter change over 215 days storage at 4 °C (
Figure 4C,D,
Table 7). An accurate model could not be developed solely using the terms of olive oil concentration, perfluorocarbon type, and their interaction. However, upon inspection of the models developed thus far, it appeared that a possible correlation may exist between nanoemulsion colloidal stability and nanoemulsion fluorescence stability. To investigate this possibility, the percent fluorescence signal loss over 215 days storage at 4 °C was included as an additional predictor variable in the MLR model for percent diameter change over 215 days storage at 4 °C. Upon addition of this term, an accurate MLR model was developed (
Figure 4C,D,
Table 7). Percent fluorescence signal loss over 215 days storage (
p-value 0.0042) significantly contributed to nanoemulsion percent diameter change over 215 days storage. Olive oil concentration (
p-value 0.0057), perfluorocarbon type (
p-value 0.0001), and the interaction between these two variables (
p-value 0.0025) also significantly contributed to nanoemulsion percent diameter change over 215 days storage. Importantly, the impacts of storage at 4 °C and incubation at 80 °C on nanoemulsion percent diameter change were comparable. Prolonged storage at 4 °C resulted in a decreased diameter in nanoemulsions containing PFOB and PFD and an increased diameter in PCE nanoemulsions. Nanoemulsions containing PFPE were the most resistant to changes in diameter upon storage at 4 °C (
Figure 4C,D). The interaction between olive oil concentration and PCE indicated that increasing olive oil concentration improves nanoemulsion colloidal stability by reducing nanoemulsion percent diameter change upon storage at 4 °C. In summary, exposing nanoemulsions to elevated temperature as a form of accelerated stability testing was determined to accurately predict nanoemulsion colloidal stability upon long-term storage at 4 °C. A correlation between nanoemulsion colloidal stability and nanoemulsion fluorescence stability was also established.
To further investigate the relationship between colloidal and fluorescence stability, an MLR model was developed to predict nanoemulsion percent fluorescence signal loss after 215 days storage at 4 °C (
Figure 4E,F,
Table 7). As was observed when modeling percent diameter change after 215 days storage, an accurate model could not be developed solely using the terms of olive oil concentration, perfluorocarbon type, and their interaction. When percent nanoemulsion diameter change after 215 days storage was included as an additional predictive term, an accurate model was developed. As was observed with the prolonged storage model of percent diameter change, fluorescence loss was found to be dependent upon olive oil concentration (
p-value 0.0155), perfluorocarbon type (
p-value 0.0044), and their interaction (
p-value 0.0134). Nanoemulsions containing PFOB and PFD experienced greater fluorescence signal loss upon prolonged storage at 4 °C when compared to nanoemulsions containing PFPE and PCE. These observations are comparable with those from the accelerated models of fluorescence stability (
Figure 2C,F), suggesting that the accelerated stability models are a good predictor of how nanoemulsions will behave over their shelf life.
3.3. Evaluation of Nanoemulsion Sedimentation with 19F Nuclear Magnetic Resonance (NMR)
In summary thus far, we identified accelerated stability tests that could be used to understand the impact of olive oil concentration and perfluorocarbon type on nanoemulsion colloidal and fluorescence stability in a time-efficient manner. Relationships detected under these accelerated conditions corresponded to nanoemulsion behavior upon prolonged (215 days) storage at 4 °C. Thus, results of the accelerated stability testing can be used to predict nanoemulsion shelf-life behavior. Further, we determined that there is a correlation between nanoemulsion colloidal stability and nanoemulsion fluorescence stability. We determined that PFOB and PFD nanoemulsions consistently underperform when compared to the PFPE and PCE nanoemulsions. As the next step in this work, we sought to develop an explanation for the clear distinctions observed between these two “groups” of nanoemulsions. While working with the nanoemulsions presented in this work, it was observed that if the nanoemulsions were left undisturbed, a thick white opaque layer would form on the bottom of the container. This observation was most apparent in nanoemulsions containing PFOB and PFD, though it was observed in all developed nanoemulsions. Upon gentle agitation, the sedimentation layer would become redispersed, and the nanoemulsion would regain a homogenous appearance. These observations were not surprising when considering the densities of the perfluorocarbons studied in this work, which range from 1.75 to 1.93 g/mL (
Table 1). Since the developed nanoemulsions contain high levels of perfluorocarbon (30%
w/
v), the density between the dispersed phase (nanoemulsion droplet) and the continuous phase (water) is significant, which can accelerate sedimentation. PFOB and PFD have comparable densities, at 1.92 and 1.93 g/mL, respectively, while PFPE and PCE have comparable densities, at 1.75 and 1.78 g/mL, respectively (
Table 1). Given this, we hypothesized that nanoemulsions containing PFOB and PFD experience sedimentation at a faster rate when compared to nanoemulsions containing PFPE or PCE. Nanoemulsion droplet sedimentation and the resulting colloidal instability leads to loss of fluorescence signal. The proposed mechanism could explain the observed correlations between nanoemulsion colloidal properties and percent fluorescent signal loss. Further, this mechanism could provide an explanation as to why the majority of the nanoemulsions presented in this work failed to meet one or more CQA specification for fluorescence (
Supplemental Table S1), while nanoemulsions previously studied by our research group demonstrated superior fluorescence stability in vitro and in vivo [
5,
8,
15]. These previously studied nanoemulsions had higher hydrocarbon content and lower perfluorocarbon content compared to the nanoemulsions studied in this work; thus, the dispersed phase of the previously studied nanoemulsions [
5,
8,
15] had a lower density, making these formulations less susceptible to sedimentation.
To test this hypothesis, nanoemulsions were left undisturbed at 4 °C for 20 days. After this time, a small volume (300 µL) was removed from the top of the nanoemulsion container, above any observed sediment. Nanoemulsions were then gently agitated to resuspend any sedimentation, and an additional 300 µL were removed. Nanoemulsion samples obtained before and after the resuspension were evaluated for diameter and PDI using DLS, and for fluorine content using
19F NMR. Diameter and PDI values measured before and after resuspension were used to calculate the percent diameter change and the change in PDI after resuspension (
Table 8). Fluorine content was quantified in the samples obtained before and after resuspension, and these values were used to calculate the perfluorocarbon percent loading of the resuspended samples and the percentage of perfluorocarbon that sedimented over the 20-day period (
Table 8). NMR spectra obtained for resuspended nanoemulsions containing 6%
w/
v olive oil are shown as an example to demonstrate how perfluorocarbon quantification was performed (
Supplemental Figure S1).
Each NMR sample was spiked with a known amount of trifluoroacetic acid (TFA). TFA has three chemically equivalent fluorine atoms that result in a peak at −76.0 ppm. The known amount of TFA in the sample and the integration areas of the TFA peak and a select perfluorocarbon peak were used to calculate the amount of perfluorocarbon in the nanoemulsion sample, and, subsequently, the perfluorocarbon percent loading and percent sedimentation. Additional details are provided in
Section 2.7. PFOB has three chemically equivalent fluorine atoms at the end of the molecule (
Figure 1) that result in a distinct peak at approximately −83.74 ppm in relation to the TFA peak (
Supplemental Figure S1A). PFD is more challenging to quantify, as it consists of two diastereomers (trans and cis). CF2 groups in the trans isomer result in four doublets that range from approximately −120 to −143 ppm in relation to the TFA peak (
Supplemental Figure S1B). A wide signal with two unresolved peaks is also observed at approximately −191.0 ppm. This signal is caused by the CF groups (
Figure 1) and is present in both PFD isomers [
16,
17]. Since this signal is caused by both isomers and, therefore, accounts for 100% of PFD in the sample, this signal was used to quantify the PFD nanoemulsion samples (
Figure 5B). PFPE has a strong peak at approximately −91.5 ppm (
Supplemental Figure S1C) that corresponds to the CF2 groups in the monomer repeating portion of the PFPE molecule (
Figure 1). The chain length is variable [
11], so an estimated resonance of 32 fluorine atoms was used in all calculations, as has been previously reported [
10]. PCE is the most straightforward perfluorocarbon to quantify. The symmetrical molecule has 20 chemically equivalent fluorine atoms (
Figure 1) that result in a single peak at approximately −92.42 ppm in relation to TFA (
Supplemental Figure S1D). NMR analysis demonstrated that nanoemulsions containing PFOB and PFD exhibit complete sedimentation of perfluorocarbon-containing droplets, as no perfluorocarbon was present in PFOB and PFD nanoemulsion samples collected prior to resuspension. PFPE and PCE nanoemulsions were found to exhibit sedimentation ranging from 21.17% (PFPE, 6%
w/
v olive oil, run 7) to 59.89% (PCE, 3%
w/
v olive oil, run 4). Across all olive oil concentrations, PFPE nanoemulsions exhibited less sedimentation than the corresponding PCE nanoemulsions. These findings are significant, as they confirm that the least stable nanoemulsions (PFOB and PFD) are the most susceptible to sedimentation, while the most stable nanoemulsions (PFPE) exhibit the lowest levels of sedimentation. Additionally, these results further strengthen our hypothesis that there is a strong correlation between nanoemulsion colloidal stability and nanoemulsion fluorescence stability.
Nanoemulsion diameter and PDI were measured before and after resuspension to indirectly measure the extent of nanoemulsion sedimentation (
Table 8). As large droplets sediment, the remaining droplets will have a smaller average diameter, and the nanoemulsion will become more homogenous, resulting in reduced PDI. PFPE nanoemulsions had the smallest values for percent diameter change and change in PDI. Accordingly, PFPE nanoemulsions also experienced the least sedimentation. PFOB and PFD nanoemulsions exhibited the largest changes in PDI, which corresponds to the complete sedimentation observed in these formulations. Interestingly, when comparing PCE nanoemulsions before and after resuspension, these formulations were found to have the largest percent diameter changes, but PDI did not increase upon resuspension. PCE nanoemulsion PDI remained comparable to the baseline value or decreased upon resuspension. The diameter and PDI changing trends were also observed when evaluating PCE nanoemulsions after exposure to accelerated stability testing or after 215 days storage at 4 °C (
Figure 3 and
Figure 4). Increasing diameter with minimal change in PDI is consistent with Ostwald ripening, which is the most common mechanism of nanoemulsion destabilization [
18]. In Ostwald ripening, large nanoemulsion droplets increase in diameter at the expense of smaller droplets. As the number of large droplets increases, sedimentation or creaming can occur [
18]. Based upon the observations in this work, we propose that PCE nanoemulsion destabilization is driven by Ostwald ripening, followed by sedimentation, while PFOB and PFD nanoemulsion destabilization is driven primarily by sedimentation alone. Nanoemulsion sedimentation results in loss of fluorescence signal, so nanoemulsions that experience the greatest levels of sedimentation will also exhibit the greatest fluorescence signal loss.
3.4. Select Formulation Optimization and Curcumin Incorporation
Through the work performed thus far, we determined that the developed nanoemulsions containing PFOB and PFD were susceptible to sedimentation because these two perfluorocarbons have exceptionally high densities (>1.90 g/mL). The extensive sedimentation exhibited by these formulations resulted in unstable nanoemulsions with greater fluorescence instability. Thus, nanoemulsions containing PFPE and PCE were deemed more promising candidates for additional optimization. PCE was selected for further optimization for several reasons. First, PCE is more commercially available than PFPE. Second, PCE is preferable for
19F NMR and MRI applications. Third, there is a precedent for the clinical translation of PCE-containing nanomaterials. PCE poly(lactic-co-glycolic acid) nanoparticles (PCE-PLGA NPs) have been developed for applications in
19F MRI [
19,
20], ultrasound [
19], and photoacoustics [
21]. These PCE-PLGA NPs are currently being investigated in phase II clinical trials for labeling dendritic cells during cell therapy for stage III melanoma patients (Identifier NCT02574377) [
22]. PCE nanoemulsion optimization included two stages. The first stage was used to identify a fluorescent dye that was not impacted by the presence of drug (curcumin). The second stage was used to select an appropriate oil that would maximize nanoemulsion colloidal stability, curcumin loading/stability, and fluorescent dye signal strength and stability. Nanoemulsion formulations for these two stages are summarized in
Table 9.
In stage one, two PCE nanoemulsion pairs were developed with olive oil and transcutol, one curcumin-loaded nanoemulsion (CUR-NE) and one curcumin-free nanoemulsion (DF-NE). The first pair (NE-1 and NE-2) contained the fluorescent dye DiD at a 10 µM concentration and the second nanoemulsion pair (NE-3 and NE-4) contained DiR at 10 µM.
Figure 5 highlights the chemical structures of curcumin and the fluorescent dyes incorporated into the nanoemulsions and depicts the structure of the developed PCE curcumin-loaded fluorescent nanoemulsions (
Figure 5A). PCE, which is both hydrophobic and lipophobic, comprises the core of the nanoemulsion droplet. The oil phase surrounding the PCE core solubilizes curcumin and/or the fluorescent dye(s). Non-ionic surfactants stabilize the nanoemulsion droplets in an aqueous continuous phase.
Figure 5B emphasizes the impact of fluorescent dye selection on nanoemulsion visual appearance. Curcumin-free nanoemulsions are the color of the fluorescent dye they contain, DiD (NE-2) or DiR (NE-3). The presence of curcumin adds bright yellow color to the nanoemulsion. The combination of curcumin and DiD results in a bright green color (NE-1), while the combination of curcumin and DiR is more yellow in color in comparison (NE-4). Together, this suggests that the presence of curcumin could have different impacts on each fluorescent dye. To confirm this, nanoemulsion fluorescence signal was measured at various nanoemulsion dilutions in de-ionized water to quantitatively evaluate the impact of curcumin on the fluorescence signal of DiD and DiR (
Figure 6A,B).
Figure 6A clearly shows that the presence of curcumin significantly reduces the fluorescence signal of DiD, while
Figure 6B shows that the DiR fluorescence signal is comparable between the curcumin-loaded and curcumin-free formulations.
The stability of each fluorescent dye after nanoemulsion incubation at body temperature was also evaluated (
Figure 6C,D). To accomplish this, undiluted nanoemulsion was incubated at 37 °C for 72 h. Fluorescence signal was then measured at various nanoemulsion dilutions in de-ionized water and compared to the fluorescence signal of nanoemulsion stored at 4 °C. Incubation at 37 °C for 72 h significantly reduces the fluorescence signal in both DiD (
Figure 6C) and DiR (
Figure 6D) nanoemulsions. Together, these results suggest that DiR is more suitable for incorporation into a curcumin-loaded PCE nanoemulsion, but that formulation adjustments should be conducted to improve the fluorescence stability of DiR. Therefore, in the second stage of nanoemulsion formulation development (
Table 9), DiR nanoemulsions with different oils were developed and evaluated.
In stage two of nanoemulsion formulation development, the impact of oil type on nanoemulsion colloidal stability, fluorescence stability, curcumin loading, and curcumin stability was evaluated. It was hypothesized that substitution of olive oil with miglyol may improve these properties. Therefore, a total of six curcumin-loaded nanoemulsions were developed (
Table 9). Nanoemulsions were developed with no fluorescent dyes (NE-5, NE-6), with DiR at 25 µM (NE-7, NE-8), and with both DiR at 25 µM and DiI at 10 µM (NE-9, NE-10). Three nanoemulsions were developed with olive oil (NE-5, NE-7, NE-9) and three nanoemulsions were developed with miglyol (NE-6, NE-8, NE-10).
Figure 7 highlights the impact of oil type on nanoemulsion colloidal stability. Nanoemulsion diameter and PDI were measured 48 h after production with dynamic light scattering. Size distribution by intensity plots is shown for nanoemulsions containing olive oil (
Figure 7A) and miglyol (
Figure 7B). These plots demonstrate that both olive oil and miglyol nanoemulsions can be consistently produced. The developed nanoemulsions contain 30%
w/
v PCE, which has a density of 1.78 g/mL. This could cause the developed nanoemulsions to be susceptible to sedimentation. To evaluate this possibility, nanoemulsions were stored at 4 °C undisturbed for 20 days, after which diameter and PDI were measured before and after nanoemulsion was thoroughly mixed/resuspended. Sedimentation of large nanoemulsions droplets would result in an observable reduction in diameter and/or PDI prior to resuspension. After the 20 days storage, no visible sediment was observed in any of the nanoemulsions. Further, both olive oil and miglyol nanoemulsions exhibited no significant change in diameter (
Figure 7C) or PDI (
Figure 7D) after resuspension, suggesting that nanoemulsions do not undergo significant sedimentation. This finding is highly significant, as previously developed PCE nanoemulsions experienced significant reduction in diameter upon 20 days storage (
Table 8). Partial sedimentation of these formulations was visually observed, and this observation was quantitatively confirmed with
19F NMR (
Table 8). Thus, the nanoemulsion optimization appears to have improved formulation colloidal stability.
To confirm that nanoemulsions do not experience significant sedimentation, PCE content was quantified in nanoemulsions before and after resuspension using
19F NMR (
Figure 8).
Figure 8A shows PCE loading values for each individual nanoemulsion. Additionally, the pooled average PCE loading was calculated for the three olive oil nanoemulsions (NE-5, NE-7, NE-9) and the three miglyol nanoemulsions (NE-6, NE-8, NE-10) produced during stage 2 of formulation development (
Figure 8B). A two-way ANOVA with Tukey’s multiple comparisons was performed, and it was determined that oil type had no significant impact on PCE loading, and that PCE loading did not significantly change when comparing nanoemulsions before and after resuspension. Together with the diameter and PDI data (
Figure 7C,D), this strongly suggests that nanoemulsions are colloidally stable and do not sediment upon storage. Interestingly, nanoemulsions containing miglyol were found to have a significantly lower PDI compared to olive oil nanoemulsions (two-way ANOVA,
p-value < 0.0001). Miglyol nanoemulsions had average PDI values of 0.138 and 0.137 before and after mixing, respectively, while olive oil nanoemulsions had average PDI values of 0.167 and 0.177 before and after mixing (
Figure 7D). This suggests that when compared to miglyol nanoemulsions, nanoemulsions developed with olive oil could be more susceptible to Ostwald ripening and, thus, could be more susceptible to changes in diameter and/or PDI.
To ensure maximum performance and shelf life of the final product, it is essential that nanoemulsion fluorescence signal and curcumin loading remain unchanged upon prolonged storage. To assess this, nanoemulsion DiR signal, DiI signal and curcumin loading were evaluated on days 100 and 165 post production (
Figure 9). Of the four nanoemulsions containing DiR, NE-9 was the only nanoemulsion to experience a significant decrease in DiR signal over the 65-day storage period (
Figure 9A,
p-value 0.0019, two-way t-test). Neither of the two nanoemulsions containing DiI experienced a significant change in DiI signal over the 65-day storage period (
Figure 9B). Further, no significant change in curcumin loading was observed for the six curcumin-loaded nanoemulsions over the 65-day storage period (
Figure 9C). Data pooling by oil type demonstrated that oil type does not have a significant impact on curcumin loading over the 65-day storage period (
Figure 9D). This finding is highly significant, as previously developed PCE nanoemulsion formulations experienced significant loss of fluorescence signal upon storage at 4 °C (
Supplemental Tables S1 and S2). Therefore, the nanoemulsion optimization presented in this work appears to have improved formulation fluorescence stability.
To further evaluate the impact of oil type on nanoemulsion colloidal stability, fluorescence stability, and curcumin loading, nanoemulsions were subjected to elevated temperature as a form of accelerated stability testing (
Figure 10). To assess the impact of oil type on nanoemulsion colloidal stability, undiluted nanoemulsions were incubated at 50 °C for 7 days (
Figure 10A,B). The pooled average diameter (
Figure 10A) and PDI (
Figure 10B) were calculated for the olive oil nanoemulsions (NE-5, NE-7, NE-9) and miglyol nanoemulsions (NE-6, NE-8, NE-10) after 50 °C incubation and compared to the average diameter and PDI for nanoemulsions stored at 4 °C. Incubation at 50 °C for 7 days results in a significant increase in average olive oil nanoemulsion diameter (
Figure 10A, two-way ANOVA, Tukey’s multiple comparisons,
p-value < 0.0001), while average miglyol nanoemulsion diameter does not significantly change. Incubation at 50 °C does not significantly change average PDI, regardless of oil type (
Figure 10B). These results complement previous findings that olive oil nanoemulsions have a significantly higher PDI compared to miglyol nanoemulsions (
Figure 7D). Together, these results suggest that compared to miglyol nanoemulsions, olive oil nanoemulsions are more susceptible to diameter increase via Ostwald ripening because their higher PDI value indicates a broader range of nanoemulsion droplet sizes, which accelerates Ostwald ripening.
To assess the impact of oil type on nanoemulsion fluorescent stability, undiluted nanoemulsions were incubated at 25 or at 37 °C for 72 h (
Figure 10C,E). Fluorescent signal of nanoemulsions stored at 4 °C was used as a control in these studies. DiR fluorescent signal remained unchanged for all for DiR-loaded nanoemulsions upon 25 °C incubation, but significant DiR signal loss was observed for all nanoemulsions upon 37 °C incubation (
Figure 10C). However, DiR signal loss was significantly greater in olive oil nanoemulsions (
Figure 10C, all **** indicate
p-values < 0.0001, two-way t-tests). DiI fluorescence was robust to incubation at elevated temperature. DiI signal was unaffected by incubation at 25 or 37 °C, and there was no significant difference in DiI signal when comparing olive oil NE-9 to miglyol NE-10 (
Figure 10E).
The impact of oil type on curcumin loading and curcumin stability in nanoemulsions was evaluated by exposing the undiluted nanoemulsions to body temperature (37 °C) for 72 h (
Figure 10D,F). Curcumin loading was calculated after the incubation period and compared to curcumin loading of nanoemulsion stored at 4 °C.
Figure 10D shows curcumin loading values for each individual nanoemulsion. Additionally, the pooled average curcumin loading was calculated for the three olive oil nanoemulsions (NE-5, NE-7, NE-9) and the three miglyol nanoemulsions (NE-6, NE-8, NE-10) produced during stage 2 of formulation development (
Figure 10F). A two-way ANOVA with Tukey’s multiple comparisons was performed, and it was determined that oil type does not have a significant impact on nanoemulsion curcumin percent loading at 4 °C or at 37 °C. Further, it was determined that incubation at 37 °C for 72 h does not have a significant impact on curcumin loading. Together, these results demonstrate that nanoemulsions can be consistently produced regardless of oil type, oil type does not significantly influence nanoemulsion curcumin loading, and incubation at 37 °C for 72 h does not have a significant impact on curcumin loading. These findings are not surprising, as the solubilizer transcutol, not the oil, is the primary curcumin carrier in the developed formulations. Since oil type does not have an impact on curcumin loading and stability in the developed formulations, oil type can be selected based on other properties, such as colloidal stability and/or fluorescence stability.
To summarize, a PCE nanoemulsion was successfully developed with optimal colloidal, fluorescence, and drug-loading stability. DiR fluorescent dye was selected due to its lack of interference with curcumin. Miglyol oil was selected because it was found to reduce nanoemulsion Ostwald ripening and fluorescence signal loss more effectively than olive oil. The optimized nanoemulsion demonstrated superior colloidal, fluorescence, and drug-loading stability, emphasizing our earlier conclusions that nanoemulsion colloidal and fluorescence stability are correlated.