The Science of Selecting Excipients for Dermal Self-Emulsifying Drug Delivery Systems
Abstract
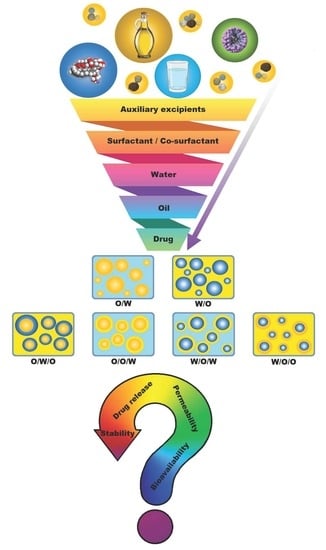
:1. Introduction
2. Components of Dermal SEDDSs/SDEDDSs
2.1. Drug(s) Inclusion
2.2. Oil Phase(s)
2.3. Surfactant(s) and Co-Surfactant(s)
2.4. The Water Phase
3. The Addition of Auxiliary Excipients
3.1. Why the Need for Refinement of Topical/Transdermal Spontaneous Emulsions?
3.2. Some Examples of Auxiliary Excipients That Can Be Included into SEDDSs and SDEDDSs for Topical/Transdermal Drug Delivery
3.2.1. Thickening Agents
3.2.2. Emollients and Humectants
3.2.3. Preservatives and Antioxidants
3.2.4. Co-Solvents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salawi, A. Self-Emulsifying Drug Delivery Systems: A Novel Approach to Deliver Drugs. Drug Deliv. 2022, 29, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Sirvi, A.; Kuche, K.; Chaudhari, D.; Ghadi, R.; Date, T.; Katiyar, S.S.; Jain, S. Supersaturable Self-Emulsifying Drug Delivery System: A Strategy for Improving the Loading and Oral Bioavailability of Quercetin. J. Drug Deliv. Sci. Technol. 2022, 71, 103289. [Google Scholar] [CrossRef]
- Lam, H.T.; Le, N.M.N.; Phan, T.N.Q.; Bernkop-Schnürch, A. Mucolytic Self-Emulsifying Drug Delivery Systems (SEDDS) Containing a Hydrophobic Ion-Pair of Proteinase. Eur. J. Pharm. Sci. 2021, 162, 105658. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, J.; Zupančič, O.; Hetényi, G.; Kurpiers, M.; Bernkop-Schnürch, A. Design and Evaluation of SEDDS Exhibiting High Emulsifying Properties. J. Drug Deliv. Sci. Technol. 2018, 44, 366–372. [Google Scholar] [CrossRef]
- van Staden, D.; du Plessis, J.; Viljoen, J. Development of a Self-Emulsifying Drug Delivery System for Optimized Topical Delivery of Clofazimine. Pharmaceutics 2020, 12, 523. [Google Scholar] [CrossRef]
- Mahmood, A.; Bernkop-Schnürch, A. SEDDS: A Game Changing Approach for the Oral Administration of Hydrophilic Macromolecular Drugs. Adv. Drug Deliv. Rev. 2019, 142, 91–101. [Google Scholar] [CrossRef]
- Almeida, S.R.D.; Tippavajhala, V.K. A Rundown Through Various Methods Used in the Formulation of Solid Self-Emulsifying Drug Delivery Systems (S-SEDDS). AAPS PharmSciTech 2019, 20, 323. [Google Scholar] [CrossRef]
- Monton, C.; Chankana, N.; Leelawat, S.; Suksaeree, J.; Songsak, T. Optimization of Supercritical Carbon Dioxide Fluid Extraction of Seized Cannabis and Self-Emulsifying Drug Delivery System for Enhancing the Dissolution of Cannabis Extract. J. Supercrit. Fluids 2022, 179, 105423. [Google Scholar] [CrossRef]
- Chudasama, A.; Patel, V.; Nivsarkar, M.; Vasu, K.; Shishoo, C.J. Role of Lipid-Based Excipients and Their Composition on the Bioavailability of Antiretroviral Self-Emulsifying Formulations. Drug Deliv. 2015, 22, 531–540. [Google Scholar] [CrossRef]
- Pouton, C.W. Lipid Formulations for Oral Administration of Drugs: Non-Emulsifying, Self-Emulsifying and ‘Self-Microemulsifying’ Drug Delivery Systems. Eur. J. Pharm. Sci. 2000, 11, S93–S98. [Google Scholar] [CrossRef]
- Wadhwa, K.; Kadian, V.; Puri, V.; Bhardwaj, B.Y.; Sharma, A.; Pahwa, R.; Rao, R.; Gupta, M.; Singh, I. New Insights into Quercetin Nanoformulations for Topical Delivery. Phytomed. Plus 2022, 2, 100257. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, R.; Huang, J.; Xia, Q. Development and Characterization of a New Non-Aqueous Self-Double-Emulsifying Drug Delivery System for Topical Application of Rutin. J. Drug Deliv. Sci. Technol. 2021, 61, 101243. [Google Scholar] [CrossRef]
- Atef, B.; Ishak, R.A.H.; Badawy, S.S.; Osman, R. Exploring the Potential of Oleic Acid in Nanotechnology-Mediated Dermal Drug Delivery: An up-to-Date Review. J. Drug Deliv. Sci. Technol. 2022, 67, 103032. [Google Scholar] [CrossRef]
- van Staden, D.; du Plessis, J.; Viljoen, J. Development of Topical/Transdermal Self-Emulsifying Drug Delivery Systems, Not as Simple as Expected. Sci. Pharm. 2020, 88, 17. [Google Scholar] [CrossRef]
- van Staden, D.; Haynes, R.K.; Viljoen, J.M. Adapting Clofazimine for Treatment of Cutaneous Tuberculosis by Using Self-Double-Emulsifying Drug Delivery Systems. Antibiotics 2022, 11, 806. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, S.; Wang, X.; Liao, J.; Yin, Z. Preparation and Evaluation of Nattokinase-Loaded Self-Double-Emulsifying Drug Delivery System. Asian J. Pharm. Sci 2015, 10, 386–395. [Google Scholar] [CrossRef]
- Khan, M.; Nadhman, A.; Sehgal, S.A.; Siraj, S.; Yasinzai, M.M. Formulation and Characterization of a Self-Emulsifying Drug Delivery System (SEDDS) of Curcumin for the Topical Application in Cutaneous and Mucocutaneous Leishmaniasis. Curr. Top. Med. Chem. 2018, 18, 1603–1609. [Google Scholar] [CrossRef]
- Forouz, F.; Dabbaghi, M.; Namjoshi, S.; Mohammed, Y.; Roberts, M.S.; Grice, J.E. Development of an Oil-in-Water Self-Emulsifying Microemulsion for Cutaneous Delivery of Rose Bengal: Investigation of Anti-Melanoma Properties. Pharmaceutics 2020, 12, 947. [Google Scholar] [CrossRef]
- Nagaraja, S.; Basavarajappa, G.M.; Attimarad, M.; Pund, S. Topical Nanoemulgel for the Treatment of Skin Cancer: Proof-of-Technology. Pharmaceutics 2021, 13, 902. [Google Scholar] [CrossRef]
- Ponto, T.; Latter, G.; Luna, G.; Leite-Silva, V.R.; Wright, A.; Benson, H.A.E. Novel Self-Nano-Emulsifying Drug Delivery Systems Containing Astaxanthin for Topical Skin Delivery. Pharmaceutics 2021, 13, 649. [Google Scholar] [CrossRef]
- Kimura, M.; Shizuki, M.; Miyoshi, K.; Sakai, T.; Hidaka, H.; Takamura, H.; Matoba, T. Relationship between the Molecular Structures and Emulsification Properties of Edible Oils. Biosci. Biotechnol. Biochem. 1994, 58, 1258–1261. [Google Scholar] [CrossRef]
- Shah, V.P.; Yacobi, A.; Rәdulescu, F.Ş.; Miron, D.S.; Lane, M.E. A Science Based Approach to Topical Drug Classification System (TCS). Int. J. Pharm. 2015, 491, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Wolk, O.; Agbaria, R. Provisional In-Silico Biopharmaceutics Classification (BCS) to Guide Oral Drug Product Development. Drug Des. Dev. Ther. 2014, 8, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.P.; RǍdulescu, F.Ş.; Miron, D.S.; Yacobi, A. Commonality between BCS and TCS. Int. J. Pharm. 2016, 509, 35–40. [Google Scholar] [CrossRef]
- Dhritlahre, R.K.; Ruchika; Padwad, Y.; Saneja, A. Self-Emulsifying Formulations to Augment Therapeutic Efficacy of Nutraceuticals: From Concepts to Clinic. Trends Food Sci. Technol. 2021, 115, 347–365. [Google Scholar] [CrossRef]
- Izgelov, D.; Shmoeli, E.; Domb, A.J.; Hoffman, A. The Effect of Medium Chain and Long Chain Triglycerides Incorporated in Self-Nano Emulsifying Drug Delivery Systems on Oral Absorption of Cannabinoids in Rats. Int. J. Pharm. 2020, 580, 119201. [Google Scholar] [CrossRef]
- Lund, A.W.; Medler, T.R.; Leachman, S.A.; Coussens, L.M. Lymphatic Vessels, Inflammation, and Immunity in Skin Cancer. Cancer Discov. 2016, 6, 22–35. [Google Scholar] [CrossRef]
- Kumar, S.; Zakrewsky, M.; Chen, M.; Menegatti, S.; Muraski, J.A.; Mitragotri, S. Peptides as Skin Penetration Enhancers: Mechanisms of Action. J. Control. Release 2015, 199, 168–178. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Gupta, R.; Dwadasi, B.S.; Rai, B.; Mitragotri, S. Effect of Chemical Permeation Enhancers on Skin Permeability: In Silico Screening Using Molecular Dynamics Simulations. Sci. Rep. 2019, 9, 1456. [Google Scholar] [CrossRef]
- Lundborg, M.; Wennberg, C.L.; Narangifard, A.; Lindahl, E.; Norlén, L. Predicting Drug Permeability through Skin Using Molecular Dynamics Simulation. J. Control. Release 2018, 283, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.E. Skin Penetration Enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, F.; Ashari, S.E.; Mat Azmi, I.D.; Abdul Rahman, M.B. Recent Advances in Encapsulation of Drug Delivery (Active Substance) in Cubosomes for Skin Diseases. J. Drug Deliv. Sci. Technol. 2022, 68, 103097. [Google Scholar] [CrossRef]
- Adesina, B.S.; Bankole, Y.O. Effects of Particle Size, Applied Pressure and Pressing Time on the Yield of Oil Expressed from Almond Seed. Niger. Food J. 2013, 31, 98–105. [Google Scholar] [CrossRef]
- Gad, H.A.; Roberts, A.; Hamzi, S.H.; Gad, H.A.; Touiss, I.; Altyar, A.E.; Kensara, O.A.; Ashour, M.L. Jojoba Oil: An Updated Comprehensive Review on Chemistry, Pharmaceutical Uses, and Toxicity. Polymers 2021, 13, 1711. [Google Scholar] [CrossRef]
- Ahmad, Z. The Uses and Properties of Almond Oil. Complement. Ther. Clin. Pract. 2010, 16, 10–12. [Google Scholar] [CrossRef]
- Hu, C.; Wang, Q.; Ma, C.; Xia, Q. Non-Aqueous Self-Double-Emulsifying Drug Delivery System: A New Approach to Enhance Resveratrol Solubility for Effective Transdermal Delivery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 360–369. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, D.B.; Kim, J.I.; Kim, P.Y. In Vitro Cytotoxicity Tests on Cultured Human Skin Fibroblasts to Predict Skin Irritation Potential of Surfactants. Toxicol. In Vitro 2000, 14, 345–349. [Google Scholar] [CrossRef]
- Kohli, K.; Chopra, S.; Dhar, D.; Arora, S.; Khar, R.K. Self-Emulsifying Drug Delivery Systems: An Approach to Enhance Oral Bioavailability. Drug Discov. Today 2010, 15, 958–965. [Google Scholar] [CrossRef]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion Components Screening and Selection: A Technical Note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef]
- Kang, B.K.; Lee, J.S.; Chon, S.K.; Jeong, S.Y.; Yuk, S.H.; Khang, G.; Lee, H.B.; Cho, S.H. Development of Self-Microemulsifying Drug Delivery Systems (SMEDDS) for Oral Bioavailability Enhancement of Simvastatin in Beagle Dogs. Int. J. Pharm. 2004, 274, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.; Shah, D.O.; Schwuger, M.J. Handbook of Applied Surface and Colloid Chemistry; Wiley: Hoboken, NJ, USA, 2002; ISBN 0471490830. [Google Scholar]
- Cheng, K.C.; Khoo, Z.S.; Lo, N.W.; Tan, W.J.; Chemmangattuvalappil, N.G. Design and Performance Optimisation of Detergent Product Containing Binary Mixture of Anionic-Nonionic Surfactants. Heliyon 2020, 6, e03861. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zheng, M.; Ma, Z.; Xin, B.; Guo, R.; Xu, X. Sugar Fatty Acid Esters. In Polar Lipids: Biology, Chemistry, and Technology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 215–243. [Google Scholar] [CrossRef]
- Miller, R. Emulsifiers: Types and Uses. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 498–502. [Google Scholar] [CrossRef]
- Premlal Ranjith, H.M.; Wijewardene, U. Lipid Emulsifiers and Surfactants in Dairy and Bakery Products. In Modifying Lipids for Use in Food; Elsevier: Amsterdam, The Netherlands, 2006; pp. 393–428. [Google Scholar] [CrossRef]
- Conde, E.; Gordon, M.H.; Moure, A.; Dominguez, H. Effects of Caffeic Acid and Bovine Serum Albumin in Reducing the Rate of Development of Rancidity in Oil-in-Water and Water-in-Oil Emulsions. Food Chem. 2011, 129, 1652–1659. [Google Scholar] [CrossRef]
- Gerde, J.A.; White, P.J. Lipids. In Soybeans: Chemistry, Production, Processing, and Utilization; Elsevier: Amsterdam, The Netherlands, 2008; pp. 193–227. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of Transdermal Drug Delivery via Synergistic Action of Chemicals. Biochim. Biophys. Acta (BBA)—Biomembr. 2009, 1788, 2362–2373. [Google Scholar] [CrossRef]
- Nangia, S.; Paul, V.K.; Deorari, A.K.; Sreenivas, V.; Agarwal, R.; Chawla, D. Topical Oil Application and Trans-Epidermal Water Loss in Preterm Very Low Birth Weight Infants-a Randomized Trial. J. Trop. Pediatr. 2015, 61, 414–420. [Google Scholar] [CrossRef]
- Hartmann, A.; Bröcker, E.B.; Hamm, H. Occlusive Treatment Enhances Efficacy of Tacrolimus 0.1% Ointment in Adult Patients with Vitiligo: Results of a Placebo-Controlled 12-Month Prospective Study. Acta Derm. Venereol. 2008, 88, 474–479. [Google Scholar] [CrossRef]
- Suñer, J.; Calpena, A.C.; Clares, B.; Cañadas, C.; Halbaut, L. Development of Clotrimazole Multiple W/O/W Emulsions as Vehicles for Drug Delivery: Effects of Additives on Emulsion Stability. AAPS PharmSciTech 2017, 18, 539–550. [Google Scholar] [CrossRef]
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal Delivery Systems in Cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Schachtel, A.; Dyer, J.A.; Boos, M.D. Climate Change and Pediatric Skin Health. Int. J. Womens Dermatol. 2021, 7, 85–90. [Google Scholar] [CrossRef]
- Taylor, P.J.; van Rosendal, S.P.; Coombes, J.S.; Gordon, R.D.; Stowasser, M. Simultaneous Measurement of Aldosterone and Cortisol by High-Performance Liquid Chromatography–Tandem Mass Spectrometry: Application to Dehydration–Rehydration Studies. J. Chromatogr. B 2010, 878, 1195–1198. [Google Scholar] [CrossRef]
- Wang, T.; Miller, D.; Burczynski, F.; Gu, X. Evaluation of Percutaneous Permeation of Repellent DEET and Sunscreen Oxybenzone from Emulsion-Based Formulations in Artificial Membrane and Human Skin. Acta Pharm. Sin. B 2014, 4, 43–51. [Google Scholar] [CrossRef]
- Tsai, C.-J.; Hsu, L.-R.; Fang, J.-Y.; Lin, H.-H. Chitosan Hydrogel as a Base for Transdermal Delivery of Berberine and Its Evaluation in Rat Skin. Biol. Pharm. Bull. 1999, 22, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Jiang, R.; Benson, H.A.E.; Roberts, M.S. Can Increasing the Viscosity of Formulations Be Used to Reduce the Human Skin Penetration of the Sunscreen Oxybenzone? J. Investig. Dermatol. 2001, 117, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Witorsch, R.J.; Thomas, J.A. Personal Care Products and Endocrine Disruption: A Critical Review of the Literature. Crit. Rev. Toxicol. 2010, 40, 1–30. [Google Scholar] [CrossRef]
- Berkey, C.; Biniek, K.; Dauskardt, R.H. Predicting Hydration and Moisturizer Ingredient Effects on Mechanical Behavior of Human Stratum Corneum. Extrem. Mech. Lett. 2021, 46, 101327. [Google Scholar] [CrossRef]
- Berkey, C.; Kanno, D.; Mehling, A.; Koch, J.P.; Eisfeld, W.; Dierker, M.; Bhattacharya, S.; Dauskardt, R.H. Emollient Structure and Chemical Functionality Effects on the Biomechanical Function of Human Stratum Corneum. Int. J. Cosmet. Sci. 2020, 42, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.S. Topical Dermatological Therapy. In Small Animal Clinical Pharmacology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 546–556. [Google Scholar] [CrossRef]
- Kar, M.; Chourasiya, Y.; Maheshwari, R.; Tekade, R.K. Current Developments in Excipient Science: Implication of Quantitative Selection of Each Excipient in Product Development. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–83. [Google Scholar] [CrossRef]
- Waller, D.G.; Sampson, A.P. Skin Disorders. In Medical Pharmacology and Therapeutics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 561–568. [Google Scholar] [CrossRef]
- Klein, K.; Palefsky, I. Shampoo Formulation. In Handbook for Cleaning/Decontamination of Surfaces; Elsevier: Amsterdam, The Netherlands, 2007; Volume 1, pp. 277–304. [Google Scholar] [CrossRef]
- Elias, P.M. Optimizing Emollient Therapy for Skin Barrier Repair in Atopic Dermatitis. Ann. Allergy Asthma Immunol. 2022, 128, 505–511. [Google Scholar] [CrossRef]
- Dominguez, S.; Mackert, G.A.; Dobke, M.K. Nanotechnology to Enhance Transdermal Delivery of Hydrophilic Humectants for Improved Skin Care: A Model for Therapeutic Applications. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 919–939. [Google Scholar] [CrossRef]
- Wurzel, K.A. Glycerol. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2005; pp. 449–451. [Google Scholar] [CrossRef]
- Yee, Q.Y.; Hassim, M.H.; Chemmangattuvalappil, N.G.; Ten, J.Y.; Raslan, R. Optimization of Quality, Safety and Health Aspects in Personal Care Product Preservative Design. Process Saf. Environ. Prot. 2022, 157, 246–253. [Google Scholar] [CrossRef]
- Bensid, A.; el Abed, N.; Houicher, A.; Regenstein, J.M.; Özogul, F. Antioxidant and Antimicrobial Preservatives: Properties, Mechanism of Action and Applications in Food–a Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2985–3001. [Google Scholar] [CrossRef]
- Hrádková, I.; Kumherová, M.; Šmidrkal, J. Preservatives in Cosmetics: Regulatory Aspects and Analytical Methods. Chem. Listy 2018, 115, 175–224. [Google Scholar] [CrossRef]
- Crofton, K.M.; Paul, K.B.; DeVito, M.J.; Hedge, J.M. Short-Term in Vivo Exposure to the Water Contaminant Triclosan: Evidence for Disruption of Thyroxine. Environ. Toxicol. Pharmacol. 2007, 24, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, L.; Kejlova, K.; Rucki, M.; Chrz, J.; Kubincova, P.; Dvorakova, M.; Kolarova, H.; Jirova, D. Health Safety of Parabens Evaluated by Selected in Vitro Methods. Regul. Toxicol. Pharmacol. 2023, 137, 105307. [Google Scholar] [CrossRef] [PubMed]
- Al Bahtiti, N. Chemical Investigation and Preservative Effect of Jordanian Nigella Sativa L. Seed Oil on Date Paste. Int. J. Res. Stud. Biosci. IJRSB 2015, 3, 1–5. [Google Scholar]
- Burdock, G.A. Assessment of Black Cumin (Nigella Sativa L.) as a Food Ingredient and Putative Therapeutic Agent. Regul. Toxicol. Pharmacol. 2022, 128, 105088. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, L.; Xia, M.; Tian, C.; Wu, W.; Dong, B.; Chu, X. SEDDS Facilitate Cinnamaldehyde Crossing the Mucus Barrier: The Perspective of Mucus and Caco-2/HT29 Co-Culture Models. Int. J. Pharm. 2022, 614, 121461. [Google Scholar] [CrossRef]
- do Nascimento Damasio, D.S.; Antunes, P.A.; Lages, E.B.; Morais-Teixeira, E.D.; Vital, K.D.; Cardoso, V.N.; Fernandes, S.O.A.; Aguiar, M.G.; Ferreira, L.A.M. A New Oral Self-Emulsifying Drug Delivery System Improves the Antileishmania Efficacy of Fexinidazole in Vivo. Int. J. Pharm. 2023, 631, 122505. [Google Scholar] [CrossRef]
- Racaniello, G.F.; Knoll, P.; Jörgensen, A.M.; Arduino, I.; Laquintana, V.; Lopedota, A.A.; Bernkop-Schnürch, A.; Denora, N. Thiolation of Non-Ionic Surfactants for the Development of Lipid-Based Mucoadhesive Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2022, 179, 95–104. [Google Scholar] [CrossRef]
- Abdalla, A.; Klein, S.; Mäder, K. A New Self-Emulsifying Drug Delivery System (SEDDS) for Poorly Soluble Drugs: Characterization, Dissolution, in Vitro Digestion and Incorporation into Solid Pellets. Eur. J. Pharm. Sci. 2008, 35, 457–464. [Google Scholar] [CrossRef]
- Parhi, R.; Swain, S. Transdermal Evaporation Drug Delivery System: Concept to Commercial Products. Adv. Pharm. Bull. 2018, 8, 535–550. [Google Scholar] [CrossRef]
- Jena, A.K.; Nayak, A.K.; De, A.; Mitra, D.; Samanta, A. Development of Lamivudine Containing Multiple Emulsions Stabilized by Gum Odina. Future J. Pharm. Sci. 2018, 4, 71–79. [Google Scholar] [CrossRef]
- N’Da, D. Prodrug Strategies for Enhancing the Percutaneous Absorption of Drugs. Molecules 2014, 19, 20780–20807. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Magnusson, B.M.; Winckle, G.; Anissimov, Y.; Roberts, M.S. Determination of the Effect of Lipophilicity on the in Vitro Permeability and Tissue Reservoir Characteristics of Topically Applied Solutes in Human Skin Layers. J. Investig. Dermatol. 2003, 120, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Nafisi, S.; Maibach, H.I. Nanotechnology in Cosmetics. In Cosmetic Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 337–369. [Google Scholar]




| Dosage Form | Year of Publication | Drug | BCS 1 Drug Class | Reference |
|---|---|---|---|---|
| SDEDDS | 2015 | Nattokinase | Enzyme | [16] |
| SNEDDS 2 | 2018 | Curcumin | Class IV | [17] |
| SEDDS | 2020 | Clofazimine | Class II | [5] |
| SEDDS | 2020 | Rose bengal | Xanthene | [18] |
| N-SDEDDS 3 | 2021 | Rutin | Class II | [12] |
| Nanoemulgel (prepared from SEDDS) | 2021 | Chrysin | Class II | [19] |
| SNEDDS 2 | 2023 | Astaxanthin | Class IV | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Staden, D.; Haynes, R.K.; Viljoen, J.M. The Science of Selecting Excipients for Dermal Self-Emulsifying Drug Delivery Systems. Pharmaceutics 2023, 15, 1293. https://doi.org/10.3390/pharmaceutics15041293
van Staden D, Haynes RK, Viljoen JM. The Science of Selecting Excipients for Dermal Self-Emulsifying Drug Delivery Systems. Pharmaceutics. 2023; 15(4):1293. https://doi.org/10.3390/pharmaceutics15041293
Chicago/Turabian Stylevan Staden, Daniélle, Richard K. Haynes, and Joe M. Viljoen. 2023. "The Science of Selecting Excipients for Dermal Self-Emulsifying Drug Delivery Systems" Pharmaceutics 15, no. 4: 1293. https://doi.org/10.3390/pharmaceutics15041293
APA Stylevan Staden, D., Haynes, R. K., & Viljoen, J. M. (2023). The Science of Selecting Excipients for Dermal Self-Emulsifying Drug Delivery Systems. Pharmaceutics, 15(4), 1293. https://doi.org/10.3390/pharmaceutics15041293