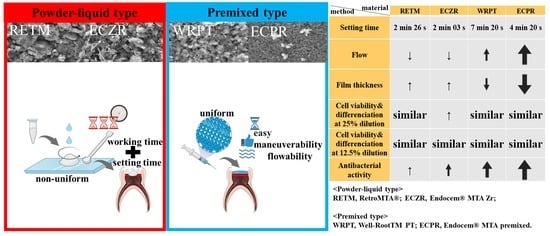
Physicochemical, Biological, and Antibacterial Properties of Four Bioactive Calcium Silicate-Based Cements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Physicochemical Analysis
2.2.1. Flow
2.2.2. Film Thickness
2.2.3. Surface Analysis
2.2.4. Crystal Structure Analysis
2.2.5. Elemental Analysis
2.2.6. Ion Release
2.2.7. pH Measurements
2.3. Biological Analysis
2.3.1. Primary Culture of Stem Cells from Human Exfoliated Deciduous Teeth (SHED)
2.3.2. Cell Viability Test
2.3.3. Odontoblastic Differentiation Test
2.4. Antibacterial Activity
2.5. Statistical Analysis
3. Results
3.1. Physicochemical Properties
3.1.1. Flow and Film Thickness
3.1.2. Surface Analysis
3.1.3. Crystal Structure
3.1.4. Elemental Analysis
3.1.5. Ion Release
3.1.6. pH
3.2. Biological Properties
3.2.1. Cell Viability
3.2.2. Odontoblastic Differentiation
3.3. Antibacterial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camilleri, J.; Pitt Ford, T. Mineral trioxide aggregate: A review of the constituents and biological properties of the material. Int. Endod. J. 2006, 39, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-I.; Choi, S.-H.; Jang, J.-H.; Chang, H.-S.; Hwang, Y.-C.; Hwang, I.-N.; Lee, B.-N.; Oh, W.-M. Effects of RetroMTA on osteoblastic differentiation in MC3T3-E1 cells. Korean J. Dent. Mater. 2018, 45, 97–109. [Google Scholar] [CrossRef]
- Kwon, Y.; Seok, S.; Lee, S.; Lim, B. Comparison of intraosseous implantation between paste type mineral trioxide aggregates (MTA) and powder-liquid mix type MTA. Korean J. Dent. Mater. 2017, 44, 229–246. [Google Scholar] [CrossRef]
- Kang, T.-Y.; Choi, J.-W.; Seo, K.-J.; Kim, K.-M.; Kwon, J.-S. Physical, chemical, mechanical, and biological properties of four different commercial root-end filling materials: A comparative study. Materials 2021, 14, 1693. [Google Scholar] [CrossRef]
- Yun, J.; You, Y.-O.; Ahn, E.; Lee, J.; An, S.-Y. Cytotoxicity of various calcium silicate-based materials with stem cells from deciduous teeth. J. Korean Acad. Pedtatri. Dent. 2019, 46, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Kunjan, A.P.; Ballal, N.V. Calcium silicate based cements in endodontics. J. Int. Dent. Med. Res. 2020, 13, 1183–1190. [Google Scholar]
- Kim, Y.; Lee, D.; Kye, M.; Ha, Y.-J.; Kim, S.-Y. Biocompatible Properties and Mineralization Potential of Premixed Calcium Silicate-Based Cements and Fast-Set Calcium Silicate-Based Cements on Human Bone Marrow-Derived Mesenchymal Stem Cells. Materials 2022, 15, 7595. [Google Scholar] [CrossRef]
- Che, J.-L.; Kim, J.-H.; Kim, S.-M.; Choi, N.-K.; Moon, H.-J.; Hwangm, M.-J.; Song, H.-J.; Park, Y.-J. Comparison of setting time, compressive strength, solubility, and pH of four kinds of MTA. Korean J. Dent. Mater. 2016, 43, 61–72. [Google Scholar] [CrossRef]
- Sharma, A.; Thomas, M.S.; Shetty, N.; Srikant, N. Evaluation of indirect pulp capping using pozzolan-based cement (ENDOCEM-Zr®) and mineral trioxide aggregate—A randomized controlled trial. J. Conserv. Dent. JCD 2020, 23, 152. [Google Scholar]
- Yamauchi, S.; Watanabe, S.; Okiji, T. Effects of heating on the physical properties of premixed calcium silicate-based root canal sealers. J. Oral Sci. 2021, 63, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Motwani, N.; Ikhar, A.; Nikhade, P.; Chandak, M.; Rathi, S.; Dugar, M.; Rajnekar, R. Premixed bioceramics: A novel pulp capping agent. J. Conserv. Dent. JCD 2021, 24, 124. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Choi, N.; Kim, S. Color Change in Tooth Induced by Various Calcium Silicate-Based Pulp-Capping Materials. J. Korean Acad. Pediatr. Dent. 2021, 48, 280–290. [Google Scholar] [CrossRef]
- Ashi, T.; Mancino, D.; Hardan, L.; Bourgi, R.; Zghal, J.; Macaluso, V.; Al-Ashkar, S.; Alkhouri, S.; Haikel, Y.; Kharouf, N. Physicochemical and Antibacterial Properties of Bioactive Retrograde Filling Materials. Bioengineering 2022, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Back, S.; Jang, Y.; Lee, J.; Lee, J.; Shin, J.; Kim, J.; Han, M.; Kim, J. pH, Ion Release Capability, and Solubility Value of Premixed Mineral Trioxide Aggregates. J. Korean Acad. Pediatr. Dent. 2022, 49, 379–391. [Google Scholar] [CrossRef]
- Kirkwood, Z.I.; Millar, B.C.; Downey, D.G.; Moore, J.E. Antimicrobial effect of dimethyl sulfoxide and N, N-Dimethylformamide on Mycobacterium abscessus: Implications for antimicrobial susceptibility testing. Int. J. Mycobacteriol. 2018, 7, 134–136. [Google Scholar]
- Ünlü, Ö.; Egil, E.; Demirci, M.; Kuşoğlu, S. Evaluation of Cytotoxicity and Antibacterial Effect of Different Types of Mineral Trioxide Aggregate. Meandros Med. Dent. J. 2023, 24, 13–18. [Google Scholar] [CrossRef]
- Chang, S.-W.; Bae, W.-J.; Yi, J.-K.; Lee, S.; Lee, D.-W.; Kum, K.-Y.; Kim, E.-C. Odontoblastic differentiation, inflammatory response, and angiogenic potential of 4 calcium silicate–based cements: Micromega MTA, ProRoot MTA, RetroMTA, and experimental calcium silicate cement. J. Endod. 2015, 41, 1524–1529. [Google Scholar] [CrossRef]
- Kwon, Y.; Seok, S.; Lee, S.; Lim, B. Comparison of physical properties between paste type mineral trioxide aggregates (MTA) and powder-liquid mix type MTA. Korean J. Dent. Mater. 2017, 44, 11–20. [Google Scholar] [CrossRef]
- ISO 10993-5: 2009; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. ISO: London, UK, 2009.
- Jang, Y.; Kim, Y.; Lee, J.; Kim, J.; Lee, J.; Han, M.R.; Kim, J.; Shin, J. Evaluation of Setting Time, Solubility, and Compressive Strength of Four Calcium Silicate-Based Cements. J. Korean Acad. Pediatr. Dent. 2023, 50, 217–228. [Google Scholar] [CrossRef]
- Baba, T.; Tsujimoto, Y. Examination of calcium silicate cements with low-viscosity methyl cellulose or hydroxypropyl cellulose additive. BioMed Res. Int. 2016, 2016, 4583854. [Google Scholar] [CrossRef] [Green Version]
- Natu, V.P.; Dubey, N.; Loke, G.C.L.; Tan, T.S.; Ng, W.H.; Yong, C.W.; Cao, T.; Rosa, V. Bioactivity, physical and chemical properties of MTA mixed with propylene glycol. J. Appl. Oral Sci. 2015, 23, 405–411. [Google Scholar] [CrossRef] [Green Version]
- ISO-6876; Dental Root Canal Sealing Materials. ISO: London, UK, 2001.
- Voicu, G.; Didilescu, A.C.; Stoian, A.B.; Dumitriu, C.; Greabu, M.; Andrei, M. Mineralogical and microstructural characteristics of two dental pulp capping materials. Materials 2019, 12, 1772. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, J.; Sorrentino, F.; Damidot, D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent. Mater. 2013, 29, 580–593. [Google Scholar] [CrossRef]
- Camilleri, J.; Cutajar, A.; Mallia, B. Hydration characteristics of zirconium oxide replaced Portland cement for use as a root-end filling material. Dent. Mater. 2011, 27, 845–854. [Google Scholar] [CrossRef]
- Viapiana, R.; Flumignan, D.; Guerreiro-Tanomaru, J.; Camilleri, J.; Tanomaru-Filho, M. Physicochemical and mechanical properties of zirconium oxide and niobium oxide modified P ortland cement-based experimental endodontic sealers. Int. Endod. J. 2014, 47, 437–448. [Google Scholar] [CrossRef]
- Kang, S.-H.; Shin, Y.-S.; Lee, H.-S.; Kim, S.-O.; Shin, Y.; Jung, I.-Y.; Song, J.S. Color changes of teeth after treatment with various mineral trioxide aggregate–based materials: An ex vivo study. J. Endod. 2015, 41, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Kodama, S.; Okiji, T. Evaluation of calcium-releasing and apatite-forming abilities of fast-setting calcium silicate-based endodontic materials. Int. Endod. J. 2015, 48, 124–130. [Google Scholar] [CrossRef]
- Borges, R.; Sousa-Neto, M.D.d.; Versiani, M.; Rached-Júnior, F.; De-Deus, G.; Miranda, C.; Pécora, J.D. Changes in the surface of four calcium silicate-containing endodontic materials and an epoxy resin-based sealer after a solubility test. Int. Endod. J. 2012, 45, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Siboni, F.; Botero, T.; Bossù, M.; Riccitiello, F.; Prati, C. Calcium silicate and calcium hydroxide materials for pulp capping: Biointeractivity, porosity, solubility and bioactivity of current formulations. J. Appl. Biomater. Funct. Mater. 2015, 13, 43–60. [Google Scholar] [CrossRef]
- Camilleri, J.; Montesin, F.E.; Brady, K.; Sweeney, R.; Curtis, R.V.; Ford, T.R.P. The constitution of mineral trioxide aggregate. Dent. Mater. 2005, 21, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Manzon, L.; Di Giorgio, R.; Orsini, G.; Tripodi, D.; Piattelli, A. Direct capping with four different materials in humans: Histological analysis of odontoblast activity. J. Endod. 2003, 29, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Mehtälä, P.; Pashley, D.; Tjäderhane, L. Effect of dimethyl sulfoxide on dentin collagen. Dent. Mater. 2017, 33, 915–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swan, A.E. Effect of Heat on the Setting Reaction and Physical Properties of Endodontic Sealers. Doctoral Dissertation, University of Illinois Chicago, Chicago, IL, USA, 2022. [Google Scholar]
- Weld, J.T.; Gunther, A. The antibacterial properties of sulfur. J. Exp. Med. 1947, 85, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Kim, E.; Song, M.; Park, J.-W.; Shin, S.-J. Effects of two fast-setting calcium-silicate cements on cell viability and angiogenic factor release in human pulp-derived cells. Odontology 2016, 104, 143–151. [Google Scholar] [CrossRef]
- Londono, S.C.; Hartnett, H.E.; Williams, L.B. Antibacterial activity of aluminum in clay from the Colombian Amazon. Environ. Sci. Technol. 2017, 51, 2401–2408. [Google Scholar] [CrossRef]
- Setbon, H.; Devaux, J.; Iserentant, A.; Leloup, G.; Leprince, J. Influence of composition on setting kinetics of new injectable and/or fast setting tricalcium silicate cements. Dent. Mater. 2014, 30, 1291–1303. [Google Scholar] [CrossRef]
- Han, W.; Sun, T.; Li, X.; Shui, Z.; Chen, Y.; Sun, M. Influence of Lithium Carbonate on C3A Hydration. Adv. Mater. Sci. Eng. 2018, 2018, 6120269. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, M.M.; Lung, C.Y.K.; Neelakantan, P.; Matinlinna, J.P. A novel, doped calcium silicate bioceramic synthesized by sol–gel method: Investigation of setting time and biological properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef]
- Francis, G.; Acharya, S.R.; Kini, S. To Determine Tooth Discolouration After Treatment with Various Endodontic Materials using Spectrophotometric Analysis-An In-Vitro Study. J. Clin. Diagn. Res. 2019, 13, 7–12. [Google Scholar] [CrossRef]
- Camilleri, J. Characterization of hydration products of mineral trioxide aggregate. Int. Endod. J. 2008, 41, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Luczaj-Cepowicz, E.; Marczuk-Kolada, G.; Pawinska, M.; Obidzinska, M.; Holownia, A. Evaluation of cytotoxicity and pH changes generated by various dental pulp capping materials—An in vitro study. Folia Histochem. Cytobiol. 2017, 55, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirose, Y.; Yamaguchi, M.; Kawabata, S.; Murakami, M.; Nakashima, M.; Gotoh, M.; Yamamoto, T. Effects of extracellular pH on dental pulp cells in vitro. J. Endod. 2016, 42, 735–741. [Google Scholar] [CrossRef]
- Torabinejad, M.; Hong, C.; Ford, T.P.; Kettering, J. Antibacterial effects of some root end filling materials. J. Endod. 1995, 21, 403–406. [Google Scholar] [CrossRef]
- Nirupama, D.N.; Nainan, M.T.; Ramaswamy, R.; Muralidharan, S.; Usha, H.H.L.; Sharma, R.; Gupta, S. In vitro evaluation of the antimicrobial efficacy of four endodontic biomaterials against Enterococcus faecalis, Candida albicans, and Staphylococcus aureus. Int. J. Biomater. 2014, 2014, 383756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özcan, E.; Yula, E.; Arslanoğlu, Z.; İnci, M. Antifungal activity of several root canal sealers against Candida albicans. Acta Odontol. Scand. 2013, 71, 1481–1485. [Google Scholar] [CrossRef]
- ElReash, A.A.; Hamama, H.; Eldars, W.; Lingwei, G.; Zaen El-Din, A.M.; Xiaoli, X. Antimicrobial activity and pH measurement of calcium silicate cements versus new bioactive resin composite restorative material. BMC Oral Health 2019, 19, 235. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.J.; Rosa, T.P.; Herrera, D.R.; Jacinto, R.C.; Gomes, B.P.; Zaia, A.A. Evaluation of cytotoxicity and physicochemical properties of calcium silicate-based endodontic sealer MTA Fillapex. J. Endod. 2013, 39, 274–277. [Google Scholar] [CrossRef]
- Sumra, Y.; Payam, S.; Zainah, I. The pH of cement-based materials: A review. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2020, 35, 908–924. [Google Scholar] [CrossRef]
- Jung, Y.; Yoon, J.-Y.; Dev Patel, K.; Ma, L.; Lee, H.-H.; Kim, J.; Lee, J.-H.; Shin, J. Biological effects of tricalcium silicate nanoparticle-containing cement on stem cells from human exfoliated deciduous teeth. Nanomaterials 2020, 10, 1373. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shin, Y.; Jung, J.; Kim, S.; Lee, J.; Song, J. Biologic response of human deciduous dental pulp cells on newly developed MTA-like materials. J. Korean Acad. Pedtatr. Dent. 2015, 42, 291–301. [Google Scholar] [CrossRef]
- Torabinejad, M.; Hong, C.; Ford, T.P.; Kettering, J. Cytotoxicity of four root end filling materials. J. Endod. 1995, 21, 489–492. [Google Scholar] [CrossRef]
- Camilleri, J.; Montesin, F.; Di Silvio, L.; Pitt Ford, T. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int. Endod. J. 2005, 38, 834–842. [Google Scholar] [CrossRef]
- Estrela, C.; Sydney, G.B.; Bammann, L.L.; Felippe Junior, O. Mechanism of the action of calcium and hydroxy ions of calcium hydroxide on tissue and bacteria. Braz. Dent. J. 1995, 6, 85–90. [Google Scholar]
- Kwon, S.-H.; Jeong, H.-J.; Lee, B.-N.; Lee, H.-S.; Kim, H.-J.; Kim, S.-Y.; Kim, D.-S.; Jang, J.-H. Effects of Various Mineral Trioxide Aggregates on Viability and Mineralization Potential of 3-Dimensional Cultured Dental Pulp Stem Cells. Appl. Sci. 2021, 11, 11381. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.-B.; Song, D.; Kim, H.-M.; Kim, S.-Y. Cytotoxicity and Mineralization Potential of Four Calcium Silicate-Based Cements on Human Gingiva-Derived Stem Cells. Coatings 2020, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, A.; Asaoka, K.; Ding, S.-J. Calcium phosphate-based cements: Clinical needs and recent progress. J. Mater. Chem. B 2013, 1, 1081–1089. [Google Scholar] [CrossRef]
- Sipert, C.R.; Hussne, R.P.; Nishiyama, C.K.; Torres, S.A. In vitro antimicrobial activity of fill canal, sealapex, mineral trioxide aggregate, Portland cement and endorez. Int. Endod. J. 2005, 38, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Al-Hezaimi, K.; Al-Shalan, T.A.; Naghshbandi, J.; Oglesby, S.; Simon, J.H.; Rotstein, I. Antibacterial effect of two mineral trioxide aggregate (MTA) preparations against Enterococcus faecalis and Streptococcus sanguis in vitro. J. Endod. 2006, 32, 1053–1056. [Google Scholar] [CrossRef]
- McHugh, C.P.; Zhang, P.; Michalek, S.; Eleazer, P.D. pH required to kill Enterococcus faecalis in vitro. J. Endod. 2004, 30, 218–219. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Giardino, L.; Palazzi, F.; Shalavi, S. Antibacterial activity of a new mineral trioxide aggregate-based root canal sealer. Int. Dent. J. 2012, 62, 70–73. [Google Scholar] [CrossRef]
- Khedmat, S.; Aminipor, M.; Pourhajibagher, M.; Kharazifar, M.J.; Bahador, A. Comparison of antibacterial activities of ProRoot MTA, OrthoMTA, and RetroMTA against three anaerobic endodontic bacteria. J. Dent. 2018, 15, 294. [Google Scholar]
- Kang, E.-H.; Yoo, J.-S.; Kim, B.-H.; Choi, S.-W.; Hong, S.-H. Synthesis and hydration behavior of calcium zirconium aluminate (Ca7ZrAl6O18) cement. Cem. Concr. Res. 2014, 56, 106–111. [Google Scholar] [CrossRef]
- Mestres, G.; Aguilera, F.S.; Manzanares, N.; Sauro, S.; Osorio, R.; Toledano, M.; Ginebra, M.-P. Magnesium phosphate cements for endodontic applications with improved long-term sealing ability. Int. Endod. J. 2014, 47, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Chang, L.; Liu, X.; Lin, J.; Liu, H.; Han, B.; Wang, S. Antibacterial property of a polyethylene glycol-grafted dental material. ACS Appl. Mater. Interfaces 2017, 9, 17688–17692. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Mun, W.; Jung, W.H.; Lee, J.; Cho, G.; Kwon, J.; Ahn, D.J.; Mitchell, R.J.; Kim, B.-S. Antimicrobial PEGtides: A modular poly (ethylene glycol)-based peptidomimetic approach to combat bacteria. ACS Nano 2021, 15, 9143–9153. [Google Scholar] [CrossRef]





| Category | Material | Abbreviation | Composition | Manufacturer | Lot Number |
|---|---|---|---|---|---|
| Powder–liquid mix type | RetroMTA® | RETM | Calcium zirconia complex, silicon dioxide, aluminum oxide, calcium carbonate, zirconium oxide | BioMTA, Seoul, Republic of Korea | RM1305D01 |
| Endocem®MTA Zr | ECZR | Natural pure cement (silicon dioxide, aluminum oxide, calcium oxide, ferric oxide, magnesium oxide), zirconium dioxide | Maruchi, Wonju, Republic of Korea | LB2103230323 | |
| Premixed type | Well-RootTM PT | WRPT | Calciumaluminosilicate compound, filler, thickening agent, zirconium oxide | Vericom, Chuncheon, Republic of Korea | WT010100 |
| Endocem® MTA premixed | ECPR | Tricalcium silicate, calcium aluminate, calcium sulfate, dimethyl sulfoxide, thickening agents (lithium carbonate, hydroxypropyl methylcellulose, phyllosilicate mineral), zirconium dioxide | Maruchi, Wonju, Republic of Korea | NWSEG110530 |
| Material | Film Thickness (µm) | Flow (mm) |
|---|---|---|
| RETM | 233.33 ± 47.25 c | 7.06 ± 0.55 a |
| ECZR | 246.66 ± 41.63 c | 7.03 ± 0.27 a |
| WRPT | 143.00 ± 13.00 b | 13.83 ± 0.35 b |
| ECPR | 30.00 ± 5.00 a | 20.18 ± 1.16 c |
| Material | Zr | Ca | C | Si | Al | O | Fe | Mg |
|---|---|---|---|---|---|---|---|---|
| RETM | 6.82 ± 0.60 | 36.93 ± 0.76 | 5.26 ± 0.21 | 4.16 ± 0.09 | 1.01 ± 0.02 | 45.82 ± 0.15 | - | - |
| ECZR | 33.90 ± 1.39 | 16.45 ± 0.35 | 3.86 ± 0.09 | 4.02 ± 0.11 | 1.85 ± 0.10 | 37.91 ± 0.97 | 1.30 ± 0.08 | 0.72 ± 0.10 |
| WRPT | 29.72 ± 3.16 | 10.62 ± 2.26 | 20.54 ± 3.52 | 2.69 ± 0.45 | 0.84 ± 0.19 | 35.58 ± 1.95 | - | - |
| ECPR | 42.18 ± 1.33 | 16.28 ± 0.36 | 3.89 ± 0.49 | 2.98 ± 0.29 | 0.40 ± 0.04 | 34.28 ± 0.43 | - | - |
| Material | Li | Sr | Ca | Al | Fe | Si | S | P |
|---|---|---|---|---|---|---|---|---|
| RETM | - | 9.40 ± 0.06 c | 195.75 ± 0.33 d | 2.60 ± 0.02 b | - | 0.46 ± 0.00 a | 0.93 ± 0.15 a | - |
| ECZR | 1.12 ± 0.01 a | 5.02 ± 0.03 b | 65.17 ± 0.54 b | 12.21 ±0.07 c | 1.26 ± 0.01 | 5.84 ± 0.01 b | 71.61 ± 0.15 c | - |
| WRPT | - | - | 1.43 ± 0.01 a | 22.76 ± 0.17 d | - | 26.02 ± 0.16 d | 26.23 ± 0.27 b | 11.55 ± 0.04 |
| ECPR | 84.69 ± 0.98 b | 2.20 ± 0.02 a | 122.26 ± 0.42 c | 0.56 ± 0.00 a | - | 7.71 ± 0.10 c | 33143.95 ± 544.80 d | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, Y.-J.; Kim, Y.-J.; Vu, H.T.; Park, J.-H.; Shin, S.-J.; Dashnyam, K.; Knowles, J.C.; Lee, H.-H.; Jun, S.-K.; Han, M.-R.; et al. Physicochemical, Biological, and Antibacterial Properties of Four Bioactive Calcium Silicate-Based Cements. Pharmaceutics 2023, 15, 1701. https://doi.org/10.3390/pharmaceutics15061701
Jang Y-J, Kim Y-J, Vu HT, Park J-H, Shin S-J, Dashnyam K, Knowles JC, Lee H-H, Jun S-K, Han M-R, et al. Physicochemical, Biological, and Antibacterial Properties of Four Bioactive Calcium Silicate-Based Cements. Pharmaceutics. 2023; 15(6):1701. https://doi.org/10.3390/pharmaceutics15061701
Chicago/Turabian StyleJang, Yu-Ji, Yu-Jin Kim, Huong Thu Vu, Jeong-Hui Park, Seong-Jin Shin, Khandmaa Dashnyam, Jonathan C. Knowles, Hae-Hyoung Lee, Soo-Kyung Jun, Mi-Ran Han, and et al. 2023. "Physicochemical, Biological, and Antibacterial Properties of Four Bioactive Calcium Silicate-Based Cements" Pharmaceutics 15, no. 6: 1701. https://doi.org/10.3390/pharmaceutics15061701
APA StyleJang, Y. -J., Kim, Y. -J., Vu, H. T., Park, J. -H., Shin, S. -J., Dashnyam, K., Knowles, J. C., Lee, H. -H., Jun, S. -K., Han, M. -R., Lee, J. -H., Kim, J. -S., Kim, J. -B., Lee, J. -H., & Shin, J. -S. (2023). Physicochemical, Biological, and Antibacterial Properties of Four Bioactive Calcium Silicate-Based Cements. Pharmaceutics, 15(6), 1701. https://doi.org/10.3390/pharmaceutics15061701