Melanoxetin: A Hydroxylated Flavonoid Attenuates Oxidative Stress and Modulates Insulin Resistance and Glycation Pathways in an Animal Model of Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Melanoxetin
2.2. Chemicals
2.3. Animal Maintenance and Experimental Design
- Male non-diabetic Wistar rats:
- ○
- Wistar non-diabetic control group (W): This group comprised male Wistar rats without DM, serving as the non-diabetic control group (n = 8).
- ○
- Wistar vehicle group (W_Vh): Male non-diabetic Wistar rats received treatment with DMSO, serving as the vehicle (n = 4).
- ○
- Wistar melanoxetin-treated group (W_M10): In this group, male non-diabetic Wistar rats received the highest administered dose of melanoxetin (10 mg/kg) (n = 3).
- Male GK rats (Type 2 DM Animal Model):
- ○
- GK control group (GK): This group consisted of male GK rats, serving as the diabetic control group (n = 6).
- ○
- GK vehicle group (GK_Vh): Male GK rats received treatment with DMSO as the vehicle (n = 8).
- ○
- GK 1 mg/kg melanoxetin group (GK_M1): Male GK rats received a dose of 1 mg/kg of melanoxetin (n = 5).
- ○
- GK 5 mg/kg melanoxetin group (GK_M5): Male GK rats received a dose of 5 mg/kg of melanoxetin (n = 8).
- ○
- GK 10 mg/kg melanoxetin group (GK_M10): Male GK rats received the highest dose of melanoxetin (10 mg/kg) (n = 6).
2.4. In Vivo Procedures and Sample Collection
2.5. Functional Studies of Aorta
2.6. Western Blotting
2.7. Histological Analysis
2.8. Determination of PGE2 Production
2.9. Statistical Analysis
3. Results
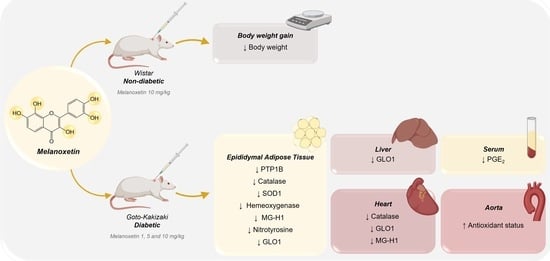
3.1. Melanotexin Reduces Body Weight in Normal Rats While Exhibiting No Alterations in Glycemia, Insulin Levels, or Pancreatic Islet Morphology in Diabetic Rats
3.2. Melanoxetin Induces Changes in PPARγ and PTP1B Expression within Epididymal Adipose Tissue
3.3. Melanoxetin Modulates Oxidative Stress and the Expression of Antioxidant Enzymes in the Epididymal Adipose and Liver Tissues of Diabetic Rats
3.4. Melanoxetin Reduces Cardiovascular Complications Associated with Diabetes by Reducing Oxidative Stress in the Heart and Increasing Acetylcholine-Dependent Vasorelaxation in the Aorta
3.5. Melanoxetin Induces the Hormetic Suppression of Serum PGE2 Levels in Diabetic Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas 2021—10th Edition. 2021. Available online: https://diabetesatlas.org/ (accessed on 13 December 2023).
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46(Suppl. S1), S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.J.; Sacre, J.W.; Harding, J.L.; Gregg, E.W.; Zimmet, P.Z.; Shaw, J.E. Young-onset type 2 diabetes mellitus—Implications for morbidity and mortality. Nat. Rev. Endocrinol. 2020, 16, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Coral, D.E.; Fernandez-Tajes, J.; Tsereteli, N.; Pomares-Millan, H.; Fitipaldi, H.; Mutie, P.M.; Atabaki-Pasdar, N.; Kalamajski, S.; Poveda, A.; Miller-Fleming, T.W.; et al. A phenome-wide comparative analysis of genetic discordance between obesity and type 2 diabetes. Nat. Metab. 2023, 5, 237–247. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers. 2015, 16, 15019. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., III. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef]
- Ma, C.-X.; Ma, X.-N.; Guan, C.-H.; Li, Y.-D.; Mauricio, D.; Fu, S.-B. Cardiovascular disease in type 2 diabetes mellitus: Progress toward personalized management. Cardiovasc. Diabetol. 2022, 21, 74. [Google Scholar] [CrossRef]
- Sharma, A.; Mittal, S.; Aggarwal, R.; Chauhan, M.K. Diabetes and cardiovascular disease: Inter-relation of risk factors and treatment. Futur. J. Pharm. Sci. 2020, 6, 130. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front. Endocrinol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Shi, Q.; Nong, K.; Vandvik, P.O.; Guyatt, G.H.; Schnell, O.; Rydén, L.; Marx, N.; Brosius, F.C.; Mustafa, R.A.; Agarwal, A.; et al. Benefits and harms of drug treatment for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2023, 381, e074068. [Google Scholar] [CrossRef] [PubMed]
- Perreault, L.; Skyler, J.S.; Rosenstock, J. Novel therapies with precision mechanisms for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.K.; Kant, S. Targeting inflammation in diabetes: Newer therapeutic options. World J. Diabetes 2014, 5, 697–710. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Martínez de Marañón, A.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: The battle continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Cooper, G.J.S. Mechanisms underlying the antidiabetic activities of polyphenolic compounds: A review. Front. Pharmacol. 2021, 12, 798329. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human study and clinical trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chang, T.-C.; Chang, S.-T. A review of antioxidant and pharmacological properties of phenolic compounds in Acacia confusa. J. Tradit. Complement. Med. 2018, 8, 443–450. [Google Scholar] [CrossRef]
- Wu, J.-H.; Tung, Y.-T.; Wang, S.-Y.; Shyur, L.-F.; Kuo, Y.-H.; Chang, S.-T. Phenolic Antioxidants from the Heartwood of Acacia confusa. J. Agric. Food Chem. 2005, 53, 5917–5921. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Chang, S.-T. Inhibition of xanthine oxidase by Acacia confusa extracts and their phytochemicals. J. Agric. Food Chem. 2010, 58, 781–786. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Hsu, C.-A.; Chen, C.-S.; Yang, S.C.; Huang, C.-C.; Chang, S.-T. Phytochemicals from Acacia confusa heartwood extracts reduce serum uric acid levels in oxonate-induced mice: Their potential use as xanthine oxidase inhibitors. J. Agric. Food Chem. 2010, 58, 9936–9941. [Google Scholar] [CrossRef]
- Wu, J.-H.; Tung, Y.-T.; Chien, S.-C.; Wang, S.-Y.; Kuo, Y.-H.; Shyur, L.-F.; Chang, S.-T. Effect of phytocompounds from the heartwood of Acacia confusa on inflammatory mediator production. J. Agric. Food Chem. 2008, 56, 1567–1573. [Google Scholar] [CrossRef]
- Proença, C.; Rufino, A.T.; Ferreira de Oliveira, J.M.P.; Freitas, M.; Fernandes, P.A.; Silva, A.M.S.; Fernandes, E. Inhibitory activity of flavonoids against human sucrase-isomaltase (α-glucosidase) activity in a Caco-2/TC7 cellular model. Food Funct. 2022, 13, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure-activity relationship study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Bodede, O.; More, G.K.; Prinsloo, G. Antimicrobial, cytotoxic and oxidative stress inhibitory activities of terpenoids and flavonols from Senegalia nigrescens (Oliv.) P.J.H. Hurter. Iran J. Pharm. Res. 2021, 20, 329–338. [Google Scholar] [PubMed]
- Sousa, J.L.C.; Proença, C.; Freitas, M.; Fernandes, E.; Silva, A.M.S. New polyhydroxylated flavon-3-ols and 3-hydroxy-2-styrylchromones: Synthesis and ROS/RNS scavenging activities. Eur. J. Med. Chem. 2016, 119, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Matafome, P.; Crisóstomo, J.; Rodrigues, L.; Fernandes, R.; Pereira, P.; Seiça, R.M. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol. Res. 2012, 65, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Akpoveso, O.-O.P.; Ubah, E.E.; Obasanmi, G. Antioxidant phytochemicals as potential therapy for diabetic complications. Antioxidants 2023, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Guest, P.C. Characterization of the Goto-Kakizaki (GK) rat model of type 2 diabetes. In Pre-Clinical Models: Techniques and Protocols; Guest, P.C., Ed.; Springer: New York, NY, USA, 2019; pp. 203–211. [Google Scholar]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. eClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Lucas, M.; Silva, V.L.M.; Gomes, P.M.O.; Silva, A.M.S.; Araújo, A.N.; Aniceto, N.; Guedes, R.C.; Corvo, M.L.; Fernandes, E.; et al. Pyrazoles as novel protein tyrosine phosphatase 1B (PTP1B) inhibitors: An in vitro and in silico study. Int. J. Biol. Macromol. 2021, 181, 1171–1182. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and obesity: Role and clinical implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Proença, C.; Ribeiro, D.; Freitas, M.; Carvalho, F.; Fernandes, E. A comprehensive review on the antidiabetic activity of flavonoids targeting PTP1B and DPP-4: A structure-activity relationship analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4095–4151. [Google Scholar] [CrossRef]
- Matafome, P.; Seiça, R. Function and Dysfunction of Adipose Tissue. In Obesity and Brain Function; Letra, L., Seiça, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–31. [Google Scholar]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Ha, H. Reactive oxygen species and oxidative stress. In Diabetes and the Kidney; Lai, K.N., Tang, S.C.W., Eds.; S.Karger AG: Basel, Switzerland, 2011; pp. 102–112. [Google Scholar]
- Ceriello, A.; Mercuri, F.; Quagliaro, L.; Assaloni, R.; Motz, E.; Tonutti, L.; Taboga, C. Detection of nitrotyrosine in the diabetic plasma: Evidence of oxidative stress. Diabetologia 2001, 44, 834–838. [Google Scholar]
- Ceriello, A.; Quagliaro, L.; Catone, B.; Pascon, R.; Piazzola, M.; Bais, B.; Marra, G.; Tonutti, L.; Taboga, C.; Motz, E. Role of hyperglycemia in nitrotyrosine Postprandial Generation. Diabetes Care 2002, 25, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced glycation end products and diabetes mellitus: Mechanisms and perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Waqas, K.; Muller, M.; Koedam, M.; el Kadi, Y.; Zillikens, M.C.; van der Eerden, B.C.J. Methylglyoxal—An advanced glycation end products (AGEs) precursor—Inhibits differentiation of human MSC-derived osteoblasts in vitro independently of receptor for AGEs (RAGE). Bone 2022, 164, 116526. [Google Scholar] [CrossRef]
- Rabbani, N. Methylglyoxal and glyoxalase 1—A metabolic stress pathway-linking hyperglycemia to the unfolded protein response and vascular complications of diabetes. Clin. Sci. 2022, 136, 819–824. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Fenske, R.J.; Weeks, A.M.; Daniels, M.; Nall, R.; Pabich, S.; Brill, A.L.; Peter, D.C.; Punt, M.; Cox, E.D.; Davis, D.B.; et al. Plasma prostaglandin e2 metabolite levels predict type 2 diabetes status and one-year therapeutic response independent of clinical markers of inflammation. Metabolites 2022, 12, 1234. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-H.; Hsiao, G.; Al-Shabrawey, M. Eicosanoids and oxidative stress in diabetic retinopathy. Antioxidants 2020, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Onodera, Y.; Teramura, T.; Takehara, T.; Shigi, K.; Fukuda, K. Reactive oxygen species induce Cox-2 expression via TAK1 activation in synovial fibroblast cells. FEBS Open Bio. 2015, 5, 492–501. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, S.; Amaro, A.; Ferreira-Junior, M.D.; Proença, C.; Silva, A.M.S.; Costa, V.M.; Oliveira, S.; Fonseca, D.A.; Silva, S.; Corvo, M.L.; et al. Melanoxetin: A Hydroxylated Flavonoid Attenuates Oxidative Stress and Modulates Insulin Resistance and Glycation Pathways in an Animal Model of Type 2 Diabetes Mellitus. Pharmaceutics 2024, 16, 261. https://doi.org/10.3390/pharmaceutics16020261
Rocha S, Amaro A, Ferreira-Junior MD, Proença C, Silva AMS, Costa VM, Oliveira S, Fonseca DA, Silva S, Corvo ML, et al. Melanoxetin: A Hydroxylated Flavonoid Attenuates Oxidative Stress and Modulates Insulin Resistance and Glycation Pathways in an Animal Model of Type 2 Diabetes Mellitus. Pharmaceutics. 2024; 16(2):261. https://doi.org/10.3390/pharmaceutics16020261
Chicago/Turabian StyleRocha, Sónia, Andreia Amaro, Marcos D. Ferreira-Junior, Carina Proença, Artur M. S. Silva, Vera M. Costa, Sara Oliveira, Diogo A. Fonseca, Sónia Silva, Maria Luísa Corvo, and et al. 2024. "Melanoxetin: A Hydroxylated Flavonoid Attenuates Oxidative Stress and Modulates Insulin Resistance and Glycation Pathways in an Animal Model of Type 2 Diabetes Mellitus" Pharmaceutics 16, no. 2: 261. https://doi.org/10.3390/pharmaceutics16020261
APA StyleRocha, S., Amaro, A., Ferreira-Junior, M. D., Proença, C., Silva, A. M. S., Costa, V. M., Oliveira, S., Fonseca, D. A., Silva, S., Corvo, M. L., Freitas, M., Matafome, P., & Fernandes, E. (2024). Melanoxetin: A Hydroxylated Flavonoid Attenuates Oxidative Stress and Modulates Insulin Resistance and Glycation Pathways in an Animal Model of Type 2 Diabetes Mellitus. Pharmaceutics, 16(2), 261. https://doi.org/10.3390/pharmaceutics16020261