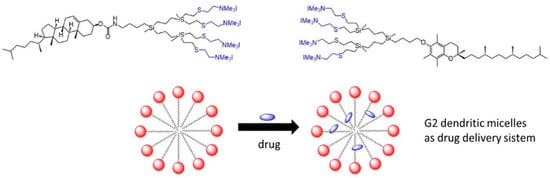
Carbosilane Dendritic Amphiphiles from Cholesterol or Vitamin E for Micelle Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical and Spectroscopic Techniques
2.3. Protocols for Drug Encapsulation
2.4. Biological Experiments
2.5. Synthesis and Characterization
3. Results and Discussion
3.1. Synthesis of Amphiphilic Dendrons
3.1.1. Cationic Dendrons with Cholesterol at the Focal Point
3.1.2. Cationic Dendrons with d-α-Tocopherol at the Periphery
3.2. Micelle Formation
3.3. Drug Encapsulation
3.4. Toxicicy Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haberland, M.E.; Reynolds, J.A. Self-association of cholesterol in aqueous solution. Proc. Natl. Acad. Sci. USA 1973, 70, 2313. [Google Scholar] [CrossRef]
- Mandal, D.; Das, S. Glucose-triggered dissolution of phenylboronic acid-functionalized cholesterol-based niosomal self-assembly for tuneable drug release. New J. Chem. 2019, 43, 7855. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, C.Y.; Wu, J.; Zhou, H.; Bai, R.; Shen, Z.; Deng, F.; Liu, Y.; Liu, J. PEG-Detachable Polymeric Micelles Self-Assembled from Amphiphilic Copolymers for Tumor-Acidity-Triggered Drug Delivery and Controlled Release. ACS Appl. Mater. Interfaces 2019, 11, 5701. [Google Scholar] [CrossRef]
- Liu, M.; Luo, X.; Qiu, Q.; Kang, L.; Li, T.; Ding, J.; Xiong, Y.; Zhao, Z.; Zan, J.; Chang, C.; et al. Redox- and pH-Sensitive Glycan (Polysialic Acid) Derivatives and F127 Mixed Micelles for Tumor-Targeted Drug Delivery. Mol. Pharm. 2018, 15, 5534. [Google Scholar] [CrossRef]
- Ferrer-Tasies, L.; Moreno-Calvo, E.; Cano-Sarabia, M.; Aguilella-Arzo, M.; Angelova, A.; Lesieur, S.; Ricart, S.; Faraudo, J.; Ventosa, N.; Veciana, J. Quatsomes: Vesicles Formed by Self-Assembly of Sterols and Quaternary Ammonium Surfactants. Langmuir 2013, 29, 6519. [Google Scholar] [CrossRef] [PubMed]
- Barnard, A.; Posocco, P.; Pricl, S.; Calderon, M.; Haag, R.; Hwang, M.E.; Shum, V.W.T.; Pack, D.W.; Smith, D.K. Degradable Self-Assembling Dendrons for Gene Delivery: Experimental and Theoretical Insights into the Barriers to Cellular Uptake. J. Am. Chem. Soc. 2011, 133, 20288. [Google Scholar] [CrossRef] [PubMed]
- Barnard, A.; Calderon, M.; Tschiche, A.; Haag, R.; Smith, D.K. Effects of a PEG additive on the biomolecular interactions of self-assembled dendron nanostructures. Org. Biomol. Chem. 2012, 10, 8403. [Google Scholar] [CrossRef]
- Barnard, A.; Posocco, P.; Fermeglia, M.; Tschiche, A.; Calderon, M.; Pricl, S.; Smith, D.K. Double-degradable responsive self-assembled multivalent arrays—Temporary nanoscale recognition between dendrons and DNA. Org. Biomol. Chem. 2014, 12, 446. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-S.; Kao, C.-L.; Chen, Y.-P.; Fang, C.-C.; Hu, C.-C.; Chu, C.-C. Photodegradable self-assembling PAMAM dendrons for gene delivery involving dendriplex formation and phototriggered circular DNA release. New J. Chem. 2016, 40, 2601. [Google Scholar] [CrossRef]
- Ou, J.-Y.; Shih, Y.-C.; Wang, B.-Y.; Chu, C.-C. Photodegradable coumarin-derived amphiphilic dendrons for DNA binding: Self-assembly and phototriggered disassembly in water and air-water interface. Colloids Surf. B 2019, 175, 428. [Google Scholar] [CrossRef]
- Preedy, V.R.; Watson, R.R. (Eds.) The Encyclopedia of Vitamin E; CABI Publishing: Wallingford, UK, 2007; p. 962. [Google Scholar]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, S.; Lin, L.; Cao, Y.; Xie, X.; Yu, H.; Chen, M.; Li, H. Redox-sensitive Pluronic F127-tocopherol micelles: Synthesis, characterization, and cytotoxicity evaluation. Int. J. Nanomed. 2017, 12, 2635. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Guo, X.; Kong, F.; Wang, Z.; Zhu, C.; Luo, L.; Li, Q.; Yang, J.; Du, Y.; et al. Specifically Increased Paclitaxel Release in Tumor and Synergetic Therapy by a Hyaluronic Acid–Tocopherol Nanomicelle. ACS Appl. Mater. Interfaces 2017, 9, 20385. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, N.; Gong, X.; Kawashima, Y.; Cui, F.; Sun, S. Tumor-targeting micelles based on folic acid and α-tocopherol succinate conjugated hyaluronic acid for paclitaxel delivery. Colloids Surf. B Biointerfaces 2019, 177, 11. [Google Scholar] [CrossRef]
- Figueira, T.N.; Mendonça, D.A.; Gaspar, D.; Melo, M.N.; Moscona, A.; Porotto, M.; Castanho, M.A.R.B.; Veiga, A.S. Structure–Stability–Function Mechanistic Links in the Anti-Measles Virus Action of Tocopherol-Derivatized Peptide Nanoparticles. ACS Nano 2018, 12, 9855. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Y.; Chen, X.; Su, J.; Huang, C. Enhanced efficacy of baicalin-loaded TPGS polymeric micelles against periodontitis. Mater. Sci. Eng. C 2019, 101, 387. [Google Scholar] [CrossRef] [PubMed]
- Banala, V.T.; Urandur, S.; Sharma, S.; Sharma, M.; Shukla, R.P.; Marwaha, D.; Gautam, S.; Dwivedi, M.; Mishra, P.R. Targeted co-delivery of the aldose reductase inhibitor epalrestat and chemotherapeutic doxorubicin via a redox-sensitive prodrug approach promotes synergistic tumor suppression. Biomater. Sci. 2019, 7, 2889. [Google Scholar] [CrossRef]
- Dong, K.; Lei, Q.; Qi, H.; Zhang, Y.; Cui, N.; Wu, X.; Xie, L.; Yan, X.; Lu, T. Amplification of Oxidative Stress in MCF-7 Cells by a Novel pH-Responsive Amphiphilic Micellar System Enhances Anticancer Therapy. Mol. Pharm. 2019, 16, 689. [Google Scholar] [CrossRef]
- Yang, C.; Wu, T.; Qi, Y.; Zhang, Z. Recent advances in the application of vitamin E TPGS for drug delivery. Theranostics 2018, 8, 464. [Google Scholar] [CrossRef]
- Mandracchia, D.; Tripodo, G.; Trapani, A.; Ruggieri, S.; Annese, T.; Chlapanidas, T.; Trapani, G.; Ribatti, D. Inulin based micelles loaded with curcumin or celecoxib with effective anti-angiogenic activity. Eur. J. Pharm. Sci. 2016, 93, 141. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, W.; Shi, X.; Fu, F.; Fan, Y.; Shen, W.; Cao, Y.; Zhang, Q.; Qi, R. Alpha-Tocopheryl Succinate-Conjugated G5 PAMAM Dendrimer Enables Effective Inhibition of Ulcerative Colitis. Adv. Healthc. Mater. 2017, 6, 1700276. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, L.; Wen, S.; Tang, Y.; Shen, M.; Zhang, G.; Shi, X. Targeted cancer theranostics using alpha-tocopheryl succinate-conjugated multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials 2014, 35, 7635. [Google Scholar] [CrossRef]
- Bhatt, H.; Kiran Rompicharla, S.V.; Ghosh, B.; Biswas, S. α-Tocopherol Succinate-Anchored PEGylated Poly(amidoamine) Dendrimer for the Delivery of Paclitaxel: Assessment of in Vitro and in Vivo Therapeutic Efficacy. Mol. Pharm. 2019, 16, 1541. [Google Scholar] [CrossRef]
- Wang, X.; Shi, C.; Zhang, L.; Bodman, A.; Guo, D.; Wang, L.; Hall, W.A.; Wilkens, S.; Luo, J. Affinity-controlled protein encapsulation into sub-30 nm telodendrimer nanocarriers by multivalent and synergistic interactions. Biomaterials 2016, 101, 258. [Google Scholar] [CrossRef]
- Wang, X.; Shi, C.; Zhang, L.; Lin, M.Y.; Guo, D.; Wang, L.; Yang, Y.; Duncan, T.M.; Luo, J. Structure-Based Nanocarrier Design for Protein Delivery. ACS Macro Lett. 2017, 6, 267. [Google Scholar] [CrossRef]
- Percec, V.; Wilson, D.A.; Leowanawat, P.; Wilson, C.J.; Hughes, A.D.; Kaucher, M.S.; Hammer, D.A.; Levine, D.H.; Kim, A.J.; Bates, F.S.; et al. Self-assembly of Janus dendrimers into uniform dendrimersomes and other complex architectures. Science 2010, 328, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Posocco, P.; Liu, X.; Cheng, Q.; Laurini, E.; Zhou, J.; Liu, C.; Wang, Y.; Tang, J.; Col, V.D.; et al. Mastering Dendrimer Self-Assembly for Efficient siRNA Delivery: From Conceptual Design to In Vivo Efficient Gene Silencing. Small 2016, 12, 3667–3676. [Google Scholar] [CrossRef]
- Kostiainen, M.A.; Kasyutich, O.; Cornelissen, J.J.L.M.; Nolte, R.J.M. Self-assembly and optically triggered disassembly of hierarchical dendron-virus complexes. Nat. Chem. 2010, 2, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.L.; Li, B.; Gillies, E.R. Surface Functionalization of Nanomaterials with Dendritic Groups: Toward Enhanced Binding to Biological Targets. J. Am. Chem. Soc. 2009, 131, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Mencia, G.; Lozano-Cruz, T.; Valiente, M.; Jimenez, J.L.; de la Mata, F.J.; Muñoz-Fernández, M.A.; Cano, J.; Gillies, E.; Gómez, R. Evaluation of pH-dependent amphiphilic carbosilane dendrons in micelle formation, drug loading and HIV-1 infection. Org. Biomol. Chem. 2020, 18, 9639–9652. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Paniagua, E.; Peña-González, C.E.; Galán, M.; Gómez, R.; de la Mata, F.J.; Sánchez-Nieves, J. Thiol-Ene Synthesis of Cationic Carbosilane Dendrons: A New Family of Synthons. Organometallics 2013, 32, 1789. [Google Scholar] [CrossRef]
- Gutiérrez-Ulloa, C.E.; Buyanova, M.Y.; Apartsin, E.K.; Venyaminova, A.G.; de la Mata, F.J.; Valiente, M.; Gómez, R. Amphiphilic carbosilane dendrons as a novel synthetic platform toward micelle formation. Org. Biomol. Chem. 2017, 15, 7352. [Google Scholar] [CrossRef] [PubMed]
- Chiappisi, L.; Keiderling, U.; Gutierrez-Ulloa, C.E.; Gómez, R.; Valiente, M.; Gradzielski, M. Aggregation behavior of surfactants with cationic and anionic dendronic head groups. J. Colloid Interf. Sci. 2019, 534, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Enache, M.; Volanschi, E. Spectral studies on the molecular interaction of anticancer drug mitoxantrone with CTBA micelles. J. Pharm. Sci. 2011, 100, 558. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, R.; Gheshlagi, M.; Tahmasebi, R.; Hojjati, F. Spectrophotometric study of interaction and solubilization of procaine hydrochloride in micellar systems. Cent. Eur. J. Chem. 2009, 7, 90–95. [Google Scholar] [CrossRef]
- Ćudina, O.; Karljiković-Rajić, K.; Ruvarac-Burgarčić, I.; Janković, I. Interaction of hydrochlorothiazide with cationic surfactant micelles of cetyltrimethylammonium bromide. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 225. [Google Scholar] [CrossRef]
- Dalpiaz, A.; Sacchetti, F.; Baldisserotto, A.; Pavan, B.; Maretti, E.; Iannuccelli, V.; Leo, E. Application of the “in-oil nanoprecipitation” method in the encapsulation of hydrophilic drugs in PLGA nanoparticles. J. Drug Deliv. Sci. Technol. 2016, 32, 283. [Google Scholar] [CrossRef]
- Sapkota, M.; Karmakar, G.; Nahak, P.; Guha, P.; Roy, B.; Koirala, S.; Chettri, P.; Das, K.; Misono, T.; Torigoe, K.; et al. Effect of polymer charge on the formation and stability of anti-inflammatory drug loaded nanostructured lipid carriers: Physicochemical approach. RSC Adv. 2015, 5, 65697. [Google Scholar] [CrossRef]
- Bhavya, R.; Upendra, N.; Jain, D.A. Development and characterization of non-ionic surfactant vesicles for ophthalmic drug delivery of diclofenac potassium. J. Drug Delivery Ther. 2014, 1–6. [Google Scholar] [CrossRef]
- Mirsharghi, S.; Knudsen, K.D.; Bagherifam, S.; Nyström, B.; Boas, U. Preparation and self-assembly of amphiphilic polylysine dendrons. New J. Chem. 2016, 40, 3597. [Google Scholar] [CrossRef]












| Dendron | CMC (μM) | Diameter (nm) | Γ (µmol/m2) | A (nm2/Molecule) | ΔG0mic (kJ/mol) |
|---|---|---|---|---|---|
| ChG2(NMe3I)4 (5) | 4.4 ± 0.7 | 9.2 ± 0.4 | 2.6 | 0.64 | −40.5 |
| ChG3(NMe3I)8 (6) | 9.0 ± 1.0 | 6.6 ± 0.1 | 2.5 | 0.65 | −38.7 |
| EG2(NMe3I)4 (17) | 5.1 ± 0.9 | 7.7 ± 0.2 | 3.1 | 0.54 | −40.1 |
| EG3(NMe3I)8 (18) | 8.0 ± 1.0 | 6.5 ± 0.3 | 2.5 | 0.67 | −39.1 |
| * C16G2(NMe3I)4 | 19 ± 1 | 5.6 ± 0.1 | 0.77 | 2.1 | - |
| * C16G3(NMe3I)8 | 17 ± 1 | 5.6 ± 0.1 | 0.69 | 2.4 | - |
| * C6G3(NMe3I)8 | 12 ± 1 | 3.8 ± 0.3 | 0,83 | 2.0 | - |
| Dendron | CMC (μM) | Diameter (nm) | Dendron | CMC (μM) | Diameter (nm) |
|---|---|---|---|---|---|
| ChG2(NMe3I)4 (5) | 4.4 ± 0.7 | 9.2 ± 0.4 | EG2(NMe3I)4 (17) | 4.8 ± 0.9 | 7.7 ± 0.2 |
| 5 + ibuprofen | 2.4 ± 0.2 | 10.0 ± 0.6 | 17 + ibuprofen | 1.22 ± 0.02 | 10.7 ± 0.4 |
| 5 + lidocaine | 2.4 ± 0.3 | 11.9 ± 0.8 | 17 + lidocaine | 3.0 ± 0.4 | 11.1 ± 0.2 |
| 5 + procaine | 2.6 ± 0.2 | 12.9 ± 0.3 | 17 + procaine | 3.7 ± 0.5 | 11.0 ± 0.7 |
| 5 + diclofenac | - | 9.1 ± 0.2 | 17 + diclofenac | - | 8.9 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mencia, G.; Algar, S.; Lozano-Cruz, T.; Muñoz-Fernández, M.Á.; Gillies, E.R.; Cano, J.; Valiente, M.; Gómez, R. Carbosilane Dendritic Amphiphiles from Cholesterol or Vitamin E for Micelle Formation. Pharmaceutics 2024, 16, 451. https://doi.org/10.3390/pharmaceutics16040451
Mencia G, Algar S, Lozano-Cruz T, Muñoz-Fernández MÁ, Gillies ER, Cano J, Valiente M, Gómez R. Carbosilane Dendritic Amphiphiles from Cholesterol or Vitamin E for Micelle Formation. Pharmaceutics. 2024; 16(4):451. https://doi.org/10.3390/pharmaceutics16040451
Chicago/Turabian StyleMencia, Gabriel, Sergio Algar, Tania Lozano-Cruz, Mª Ángeles Muñoz-Fernández, Elizabeth R. Gillies, Jesús Cano, Mercedes Valiente, and Rafael Gómez. 2024. "Carbosilane Dendritic Amphiphiles from Cholesterol or Vitamin E for Micelle Formation" Pharmaceutics 16, no. 4: 451. https://doi.org/10.3390/pharmaceutics16040451
APA StyleMencia, G., Algar, S., Lozano-Cruz, T., Muñoz-Fernández, M. Á., Gillies, E. R., Cano, J., Valiente, M., & Gómez, R. (2024). Carbosilane Dendritic Amphiphiles from Cholesterol or Vitamin E for Micelle Formation. Pharmaceutics, 16(4), 451. https://doi.org/10.3390/pharmaceutics16040451