Influence of Chronobiology on the Nanoparticle-Mediated Drug Uptake into the Brain
Abstract
:1. Introduction
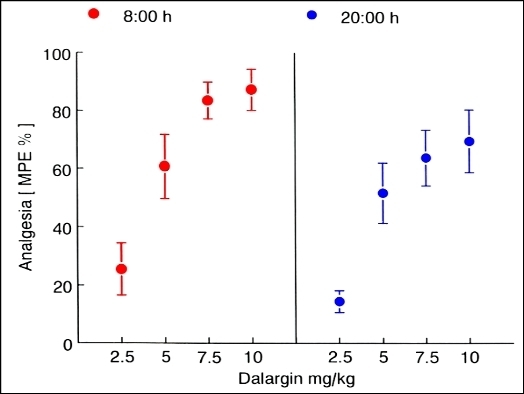
2. Circadian Influence on the Pharmacological Activity of an Analgesic Hexapeptide Delivered by Nanoparticles across the Blood–Brain Barrier


3. Conclusions
Conflicts of Interest
References
- Davson, H.; Segal, M. Physiology of the CSF and Blood–Brain Barrier; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Begley, D.J. The blood-brain barrier: Principles for targeting peptides and drugs to the central nervous system. J. Pharm. Pharmacol. 1996, 48, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Begley, D.J. Delivery of therapeutic agents to the central nervous system: The problems and possibilities. Pharmacol. Ther. 2004, 104, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv. Drug Deliv. Rev. 2014, 71, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood-brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Mechanism of polymeric nanoparticle-based drug transport across the blood-brain barrier (BBB). J. Microencapsul. 2013, 30, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Zensi, A.; Begley, D.; Pontikis, C.; Legros, C.; Mihoreanu, L.; Wagner, S.; Büchel, C.; von Briesen, H.; Kreuter, J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J. Control. Release 2009, 137, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J.; Petrov, V.E.; Kharkevich, D.A.; Alyautdin, R.N. Influence of the type of surfactant on the analgesic effects induced by the peptide dalargin after its delivery across the blood-brain barrier using surfactant-coated nanoparticles. J. Control. Release 1997, 49, 81–87. [Google Scholar] [CrossRef]
- Petri, B.; Bootz, A.; Khalansky, A.; Hekmatara, T.; Müller, R.; Uhl, R.; Kreuter, J.; Gelperina, S. Chemotherapy of brain tumour using doxorubicin bound to surfactant-coated poly(butyl cyanoacrylate) nano-particles: Revisiting the role of surfactants. J. Control. Release 2007, 117, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J.; Hekmatara, T.; Dreis, S.; Vogel, T.; Gelperina, S.; Langer, K. Covalent attachment of apolipoprotein A-I and apolipoprotein B-100 to albumin nanoparticles enables drug transport into the brain. J. Control. Release 2007, 118, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Knobloch, T.; Kreuter, J. Targeting the insulin receptor: Nanoparticles for drug delivery across the blood-brain barrier (BBB). J. Drug Target. 2011, 19, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ramge, P.; Kreuter, J.; Lemmer, B. Circadian phase-dependent antinociceptive reaction in mice after i. v. injection of dalargin-loaded nanoparticles determined by the hot-plate test and the tail-flick test. Chronobiol. Int. 1999, 17, 767–777. [Google Scholar]
- Lemmer, B. Chronobiology, drug-delivery, and chronotherapeutics. Adv. Drug Deliv. Rev. 2007, 59, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, B. Discoveries of rhythms in human biological functions: A historical review. Chronobiol. Int. 2009, 26, 1019–1068. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, B. The importance of circadian rhythms on drug response in hypertension and coronary heart disease—From mice and man. Pharmacol. Ther. 2006, 111, 629–651. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.H.; Herzog, E.D. Clock genes, oscillators, and cellular networks in the suprachiasmatic nuclei. J. Biol. Rhythm. 2004, 19, 400–413. [Google Scholar] [CrossRef]
- Dallmann, R.; Brown, S.A.; Gachon, F. Chronopharmacology: New Insights and therapeutic implications. Ann. Rev. Pharmacol. Toxicol. 2014, 54, 339–361. [Google Scholar] [CrossRef]
- Lemmer, B. Chronopharmacology and controlled drug release. Exp. Opin. Drug Deliv. 2005, 2, 667–681. [Google Scholar] [CrossRef]
- Labrecque, G.; Reinberg, A.E. Chronopharmacology of nonsteroid anti-inflammatory drugs. In Chronopharmacology-Cellular and Biochemical Interactions; Lemmer, B., Ed.; Marcel Dekker: New York, NY, USA, 1989; pp. 545–579. [Google Scholar]
- Labrecque, G.; Karzazi, M.; Vanier, M.-C. Biological rhythms in pain and analgesia. In Physiology and Pharmacology of Biological Rhythms. Handbook of Experimental Pharmacology; Redfern, P., Lemmer, B., Eds.; Springer: Heidelberg, Germany, 1997; Volume 125, pp. 619–649. [Google Scholar]
- Naber, D.; Wirz-Justice, A.; Kafka, M.S. Circadian rhythm in rat brain opiate receptor. Neurosci. Lett. 1981, 21, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, R.C.A.; Burgis, V.; Edwards, J.D. Hyperalgesia induced by naloxone follows diurnal rhythm in responsivity to painful stimuli. Science 1977, 198, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Pencheva, N.; Pospišek, J.; Hauzerova, L.; Barth, T.; Milanov, P. Activity profiles of dalargin and its analogues in μ-, δ- and κ-opioid receptor selective bioassays. Br. J. Pharmacol. 1999, 128, 569–576. [Google Scholar] [CrossRef]
- Kreuter, J.; Alyautdin, R.N.; Kharkevich, D.A.; Ivanov, A.A. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res. 1995, 674, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Alyautdin, R.; Gothier, D.; Petrov, V.; Kharkevich, D.; Kreuter, J. Analgesic activity of the hexapeptide dalargin adsorbed on the surface of polysorbate 80-coated poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 1995, 41, 44–48. [Google Scholar]
- Lück, M. Plasmaproteinadsorption als möglicher Schlüsselfaktor für eine kontrollierte Arzneistoffapplikation mit partikulären Trägern. Ph.D. Thesis, Freie Universität, Berlin, Germany, 1997; pp. 14–24, 137–154. [Google Scholar]
- Kreuter, J.; Shamenkov, D.; Petrov, V.; Ramge, P.; Cychutek, K.; Koch-Brandt, C.; Alyautdin, R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J. Drug Target. 2002, 10, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Zensi, A.; Wien, S.L.; Tschickardt, S.E.; Maier, W.; Vogel, T.; Worek, F.; Pietrzik, C.U.; Kreuter, J.; von Briesen, H. Uptake mechanism of apoE-modified nanoparticles on brain capillary endothelial cells as a blood-brain barrier model. PLoS One 2012, 7, e32568. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Jinnai, K.; Endo, M.; Fujita, K.; Kimura, F. Diurnal variation of cerebral blood flow in rat hippocampus. Stroke 1990, 21, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Wolburg, H. Mini review: Transendothelial migration of leukocytes: Through the front door or around the side of the house? Eur. J. Immunol. 2004, 34, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreuter, J. Influence of Chronobiology on the Nanoparticle-Mediated Drug Uptake into the Brain. Pharmaceutics 2015, 7, 3-9. https://doi.org/10.3390/pharmaceutics7010003
Kreuter J. Influence of Chronobiology on the Nanoparticle-Mediated Drug Uptake into the Brain. Pharmaceutics. 2015; 7(1):3-9. https://doi.org/10.3390/pharmaceutics7010003
Chicago/Turabian StyleKreuter, Jörg. 2015. "Influence of Chronobiology on the Nanoparticle-Mediated Drug Uptake into the Brain" Pharmaceutics 7, no. 1: 3-9. https://doi.org/10.3390/pharmaceutics7010003
APA StyleKreuter, J. (2015). Influence of Chronobiology on the Nanoparticle-Mediated Drug Uptake into the Brain. Pharmaceutics, 7(1), 3-9. https://doi.org/10.3390/pharmaceutics7010003