Neurosurgical Techniques for Disruption of the Blood–Brain Barrier for Glioblastoma Treatment
Abstract
:1. Introduction

2. Superselective Intra-Arterial Mannitol Infusion

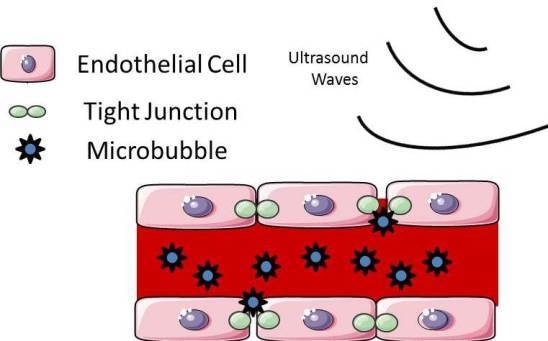
3. Focused Ultrasound

4. Laser Interstitial Thermotherapy
5. Non-Thermal Irreversible Electroporation

6. Conclusions
Author Contributions
Conflicts of Interest
References
- Dyrna, F.; Hanske, S.; Krueger, M.; Bechmann, I. The blood-brain barrier. J. Neuroimmune Pharmacol. 2013, 8, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.L.; Brites, D.; Brito, M.A. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010, 64, 328–363. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011, 14, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Mikitsh, J.L.; Chacko, A.-M. Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect. Medicin. Chem. 2014, 6, 11–24. [Google Scholar] [PubMed]
- Bauer, H.-C.; Krizbai, I.A.; Bauer, H.; Traweger, A. “You Shall Not Pass”-tight junctions of the blood brain barrier. Front. Neurosci. 2014, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Jovčevska, I.; Kočevar, N.; Komel, R. Glioma and glioblastoma - how much do we (not) know? Mol. Clin. Oncol. 2013, 1, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Paw, I.; Carpenter, R.C.; Watabe, K.; Debinski, W.; Lo, H.-W. Mechanisms regulating glioma invasion. Cancer Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, G.F.; Dunn, G.P.; Nance, E.A.; Hanes, J.; Brem, H. Emerging insights into barriers to effective brain tumor therapeutics. Front. Oncol. 2014, 4, 126. [Google Scholar] [CrossRef] [PubMed]
- Aiken, R. Molecular neuro-oncology and the challenge of the blood-brain barrier. Semin. Oncol. 2014, 41, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.D.; Price, J.E.; Fujimaki, T.; Bucana, C.D.; Fidler, I.J. Differential permeability of the blood-brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am. J. Pathol. 1992, 141, 1115–1124. [Google Scholar] [PubMed]
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 4196. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.C. Blood-brain barrier breakdown in septic encephalopathy and brain tumours. J. Anat. 2002, 200, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S.; Binder, D.K.; Manley, G.T.; Krishna, S.; Verkman, A.S. Molecular mechanisms of brain tumor edema. Neuroscience 2004, 129, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.G.; Campanati, L.; Righy, C.; D’Andrea-Meira, I.; de Sampaio e Spohr, T.C.L.; Porto-Carreiro, I.; Pereira, C.M.; Balça-Silva, J.; Kahn, S.A.; DosSantos, M.F.; et al. Gliomas and the vascular fragility of the blood brain barrier. Front. Cell. Neurosci. 2014, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Frenkel, E.P.; Neuwelt, E.A. The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology 2011, 76, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Van Vulpen, M.; Kal, H.B.; Taphoorn, M.J.B.; El-Sharouni, S.Y. Changes in blood-brain barrier permeability induced by radiotherapy: implications for timing of chemotherapy? Oncol. Rep. 2002, 9, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, J.-K.; Riina, H.; Shin, B.J.; Christos, P.; Kesavabhotla, K.; Hofstetter, C.P.; Tsiouris, A.J.; Boockvar, J.A. Intra-arterial delivery of bevacizumab after blood-brain barrier disruption for the treatment of recurrent glioblastoma: Progression-free survival and overall survival. World Neurosurg. 2012, 77, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Bidros, D.S.; Vogelbaum, M.A. Novel drug delivery strategies in neuro-oncology. Neurotherapeutics 2009, 6, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Miller, G. Drug targeting. Breaking down barriers. Science 2002, 297, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Chi, O.Z.; Wei, H.M.; Lu, X.; Weiss, H.R. Increased blood-brain permeability with hyperosmolar mannitol increases cerebral O2 consumption and O2 supply/consumption heterogeneity. J. Cereb. Blood Flow Metab. 1996, 16, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Ellis, J.A.; Emala, C.W. Revisiting intra-arterial drug delivery for treating brain diseases or is it “déjà-vu, all over again”? J. Neuroanaesth. Crit. Care 2014, 1, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.J.; Burkhardt, J.-K.; Riina, H.A.; Boockvar, J.A. Superselective intra-arterial cerebral infusion of novel agents after blood-brain disruption for the treatment of recurrent glioblastoma multiforme: A technical case series. Neurosurg. Clin. N. Am. 2012, 23, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Boockvar, J.A.; Tsiouris, A.J.; Hofstetter, C.P.; Kovanlikaya, I.; Fralin, S.; Kesavabhotla, K.; Seedial, S.M.; Pannullo, S.C.; Schwartz, T.H.; Stieg, P.; et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. J. Neurosurg. 2011, 114, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Riina, H.A.; Knopman, J.; Greenfield, J.P.; Fralin, S.; Gobin, Y.P.; Tsiouris, A.J.; Souweidane, M.M.; Boockvar, J.A. Balloon-assisted superselective intra-arterial cerebral infusion of bevacizumab for malignant brainstem glioma. A technical note. Interv. Neuroradiol. 2010, 16, 71–76. [Google Scholar] [PubMed]
- Yamane, T.; Kaneko, A.; Mohri, M. The technique of ophthalmic arterial infusion therapy for patients with intraocular retinoblastoma. Int. J. Clin. Oncol. 2004, 9, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, P.; Chalouhi, N.; Tjoumakaris, S.; Gonzalez, L.F.; Dumont, A.S.; Chitale, R.; Rosenwasser, R.; Bianciotto, C.G.; Shields, C. Pearls and pitfalls of intraarterial chemotherapy for retinoblastoma. J. Neurosurg. Pediatr. 2012, 10, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, K. Ultrasound for drug and gene delivery to the brain. Adv. Drug Deliv. Rev. 2008, 60, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.G.; Roy, R.A. Measurements of bubble-enhanced heating from focused, MHz-frequency ultrasound in a tissue-mimicking material. Ultrasound Med. Biol. 2001, 27, 1399–1412. [Google Scholar] [CrossRef]
- Kim, Y.; Rhim, H.; Choi, M.J.; Lim, H.K.; Choi, D. High-intensity focused ultrasound therapy: An overview for radiologists. Korean J. Radiol. 2008, 9, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef] [PubMed]
- McDannold, N.J.; Vykhodtseva, N.I.; Hynynen, K. Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology 2006, 241, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Sheikov, N.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.; Hynynen, K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med. Biol. 2004, 30, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Konofagou, E.E. The size of blood-brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure. J. Cereb. Blood Flow Metab. 2014, 34, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-Y.; Ko, C.-E.; Huang, S.-Y.; Chung, I.-F.; Chen, G.-S. Pharmacokinetic changes induced by focused ultrasound in glioma-bearing rats as measured by dynamic contrast-enhanced MRI. PLoS ONE 2014, 9, e92910. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Vykhodtseva, N.; Zhang, Y.-Z.; Park, J.; McDannold, N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J. Control. Release 2013, 169, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Huang, C.-Y.; Chen, J.-Y.; Wang, H.-Y.J.; Chen, P.-Y.; Wei, K.-C. Pharmacodynamic and therapeutic investigation of focused ultrasound-induced blood-brain barrier opening for enhanced temozolomide delivery in glioma treatment. PLoS ONE 2014, 9, e114311. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-Y.; Wong, T.-T.; Teng, M.-C.; Liu, R.-S.; Lu, M.; Liang, H.-F.; Wei, M.-C. Focused ultrasound and interleukin-4 receptor-targeted liposomal doxorubicin for enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme. J. Control. Release 2012, 160, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J.; McVeigh, P.Z.; O’Reilly, M.A.; Burrell, K.; Bebenek, M.; Smith, C.; Etame, A.B.; Zadeh, G.; Hynynen, K.; Wilson, B.C.; et al. Focused ultrasound delivery of Raman nanoparticles across the blood-brain barrier: Potential for targeting experimental brain tumors. Nanomedicine 2014, 10, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Huber, P.E.; Pfisterer, P. In vitro and in vivo transfection of plasmid DNA in the Dunning prostate tumor R3327-AT1 is enhanced by focused ultrasound. Gene Ther. 2000, 7, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem. Biophys. Res. Commun. 2006, 340, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A. Ultrasound-induced blood-brain barrier opening for drug delivery. Front. Neurol. Neurosci. 2015, 36, 106–115. [Google Scholar] [PubMed]
- McDannold, N.; Clement, G.T.; Black, P.; Jolesz, F.; Hynynen, K. Transcranial magnetic resonance imaging-guided focused ultrasound surgery of brain tumors: Initial findings in 3 patients. Neurosurgery 2010, 66, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Coluccia, D.; Fandino, J.; Schwyzer, L.; O’Gorman, R.; Remonda, L.; Anon, J.; Martin, E.; Werner, B. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J. Ther. Ultrasound 2014, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Iancu, C.; Mocan, L. Advances in cancer therapy through the use of carbon nanotube-mediated targeted hyperthermia. Int. J. Nanomedicine 2011, 6, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Stafford, R.J.; Fuentes, D.; Elliott, A.A.; Weinberg, J.S.; Ahrar, K. Laser-induced thermal therapy for tumor ablation. Crit. Rev. Biomed. Eng. 2010, 38, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Jethwa, P.R.; Barrese, J.C.; Gowda, A.; Shetty, A.; Danish, S.F. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: Initial experience. Neurosurgery 2012, 71, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Sloan, A.E.; Ahluwalia, M.S.; Valerio-Pascua, J.; Manjila, S.; Torchia, M.G.; Jones, S.E.; Sunshine, J.L.; Phillips, M.; Griswold, M.A.; Clampitt, M.; et al. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma: Clinical article. J. Neurosurg. 2013, 118, 1202–1219. [Google Scholar] [CrossRef] [PubMed]
- Torres-Reveron, J.; Tomasiewicz, H.C.; Shetty, A.; Amankulor, N.M.; Chiang, V.L. Stereotactic laser induced thermotherapy (LITT): A novel treatment for brain lesions regrowing after radiosurgery. J. Neurooncol. 2013, 113, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; McNichols, R.J.; Stafford, R.J.; Itzcovitz, J.; Guichard, J.-P.; Reizine, D.; Delaloge, S.; Vicaut, E.; Payen, D.; Gowda, A.; et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery 2008, 63, 21–29. [Google Scholar] [CrossRef]
- Carpentier, A.; Chauvet, D.; Reina, V.; Beccaria, K.; Leclerq, D.; McNichols, R.J.; Gowda, A.; Cornu, P.; Delattre, J.-Y. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg. Med. 2012, 44, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Lee Titsworth, W.; Murad, G.J.A.; Hoh, B.L.; Rahman, M. Fighting fire with fire: The revival of thermotherapy for gliomas. Anticancer Res. 2014, 34, 565–574. [Google Scholar] [PubMed]
- Hawasli, A.H.; Ray, W.Z.; Murphy, R.K.J.; Dacey, R.G.; Leuthardt, E.C. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for subinsular metastatic adenocarcinoma: Technical case report. Neurosurgery 2012, 70, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.M.; Hawasli, A.H.; Rodriguez, A.; Schroeder, J.L.; Laxton, A.W.; Elson, P.; Tatter, S.B.; Barnett, G.H.; Leuthardt, E.C. The Role of Laser Interstitial Thermal Therapy in Enhancing Progression Free Survival of Difficult-to-Access High Grade Gliomas: A Multi-Center Study. Cancer 2014, 3, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Schober, R.; Bettag, M.; Sabel, M.; Ulrich, F.; Hessel, S. Fine structure of zonal changes in experimental Nd:YAG laser-induced interstitial hyperthermia. Lasers Surg. Med. 1993, 13, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Hawasli, A.H.; Kim, A.H.; Dunn, G.P.; Tran, D.D.; Leuthardt, E.C. Stereotactic laser ablation of high-grade gliomas. Neurosurg. Focus 2014, 37, E1. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Matsumoto, K.; Higashi, H.; Furuta, T.; Ohmoto, T. Acute effects of interstitial hyperthermia on normal monkey brain--magnetic resonance imaging appearance and effects on blood-brain barrier. Neurol. Med. Chir. 1994, 34, 668–675. [Google Scholar] [CrossRef]
- Tsong, T.Y. Electroporation of cell membranes. Biophys. J. 1991, 60, 297–306. [Google Scholar] [CrossRef]
- Golberg, A.; Yarmush, M.L. Nonthermal irreversible electroporation: Fundamentals, applications, and challenges. IEEE Trans. Biomed. Eng. 2013, 60, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.L.; Garcia, P.A.; Rossmeisl, J.H.; Henao-Guerrero, N.; Robertson, J.; Davalos, R.V. Nonthermal irreversible electroporation for intracranial surgical applications. Laboratory investigation. J. Neurosurg. 2011, 114, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Hjouj, M.; Last, D.; Guez, D.; Daniels, D.; Sharabi, S.; Lavee, J.; Rubinsky, B.; Mardor, Y. MRI study on reversible and irreversible electroporation induced blood brain barrier disruption. PLoS ONE 2012, 7, e42817. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.A.; Rossmeisl, J.H.; Robertson, J.L.; Olson, J.D.; Johnson, A.J.; Ellis, T.L.; Davalos, R.V. 7.0-T magnetic resonance imaging characterization of acute blood-brain-barrier disruption achieved with intracranial irreversible electroporation. PLoS ONE 2012, 7, e50482. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, J.H.; Garcia, P.A.; Roberston, J.L.; Ellis, T.L.; Davalos, R.V. Pathology of non-thermal irreversible electroporation (N-TIRE)-induced ablation of the canine brain. J. Vet. Sci. 2013, 14, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.A.; Pancotto, T.; Rossmeisl, J.H.; Henao-Guerrero, N.; Gustafson, N.R.; Daniel, G.B.; Robertson, J.L.; Ellis, T.L.; Davalos, R.V. Non-thermal irreversible electroporation (N-TIRE) and adjuvant fractionated radiotherapeutic multimodal therapy for intracranial malignant glioma in a canine patient. Technol. Cancer Res. Treat. 2011, 10, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, M.; Singh, A.V. Nanoparticle enabled drug delivery across the blood brain barrier: In vivo and in vitro models, opportunities and challenges. Curr. Pharm. Biotechnol. 2014, 14, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Wang, Z.; Liu, N.; Lin, W.; Wang, X.; Xu, D.; Liu, H.; Zeng, C.; Xie, X.; Mei, X.; et al. Improving efficiency of adriamycin crossing blood brain barrier by combination of thermosensitive liposomes and hyperthermia. Biol. Pharm. Bull. 2011, 34, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.P.; Rubin, D.G.; Santillan, A.; Sondhi, D.; Dyke, J.P.; Pierre Gobin, Y.; Crystal, R.G.; Ballon, D.J. Intra-arterial delivery of AAV vectors to the mouse brain after mannitol mediated blood brain barrier disruption. J. Control. Release 2014, 196, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Al-Shehri, A.; Favretto, M.E.; Ioannou, P.V.; Romero, I.A.; Couraud, P.-O.; Weksler, B.B.; Parker, T.L.; Kallinteri, P. Permeability of PEGylated Immunoarsonoliposomes Through In Vitro Blood Brain Barrier-Medulloblastoma Co-culture Models for Brain Tumor Therapy. Pharm. Res. 2015, 32, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Bicker, J.; Alves, G.; Fortuna, A.; Falcão, A. Blood-brain barrier models and their relevance for a successful development of CNS drug delivery systems: A review. Eur. J. Pharm. Biopharm. 2014, 87, 409–432. [Google Scholar] [CrossRef] [PubMed]
- Chassidim, Y.; Vazana, U.; Prager, O.; Veksler, R.; Bar-Klein, G.; Schoknecht, K.; Fassler, M.; Lublinsky, S.; Shelef, I. Analyzing the blood-brain barrier: The benefits of medical imaging in research and clinical practice. Semin. Cell Dev. Biol. 2015, 38, 43–52. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, A.; Tatter, S.B.; Debinski, W. Neurosurgical Techniques for Disruption of the Blood–Brain Barrier for Glioblastoma Treatment. Pharmaceutics 2015, 7, 175-187. https://doi.org/10.3390/pharmaceutics7030175
Rodriguez A, Tatter SB, Debinski W. Neurosurgical Techniques for Disruption of the Blood–Brain Barrier for Glioblastoma Treatment. Pharmaceutics. 2015; 7(3):175-187. https://doi.org/10.3390/pharmaceutics7030175
Chicago/Turabian StyleRodriguez, Analiz, Stephen B. Tatter, and Waldemar Debinski. 2015. "Neurosurgical Techniques for Disruption of the Blood–Brain Barrier for Glioblastoma Treatment" Pharmaceutics 7, no. 3: 175-187. https://doi.org/10.3390/pharmaceutics7030175
APA StyleRodriguez, A., Tatter, S. B., & Debinski, W. (2015). Neurosurgical Techniques for Disruption of the Blood–Brain Barrier for Glioblastoma Treatment. Pharmaceutics, 7(3), 175-187. https://doi.org/10.3390/pharmaceutics7030175