Image-Guided Hydrodynamic Gene Delivery: Current Status and Future Directions
Abstract
:1. Hydrodynamics-Based Gene Delivery in Small Animals

2. Experimental Applicability of Hydrodynamic Gene Delivery
3. Clinical Applicability of Image-Guided Hydrodynamic Gene Delivery


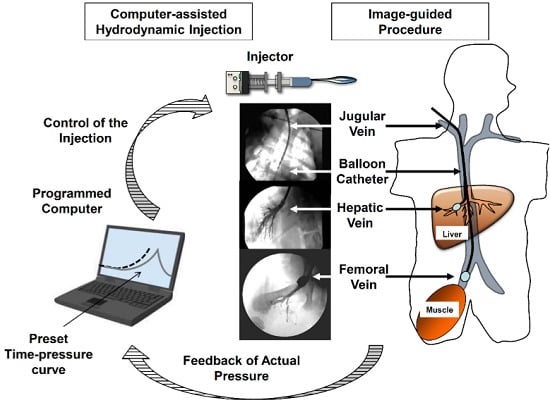
4. Clinically Applicable Reproducible Procedure
5. Conclusions and Future Perspectives
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Liu, F.; Song, Y.; Liu, D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999, 6, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Budker, V.; Wolff, J.A. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum. Gene Ther. 1999, 10, 1735–1737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Gao, X.; Song, Y.K.; Vollmer, R.; Stolz, D.B.; Gasiorowski, J.Z.; Dean, D.A.; Liu, D. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004, 11, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Song, Y.K.; Liu, D. Long-term expression of human alpha1-antitrypsin gene in mouse liver achieved by intravenous administration of plasmid DNA using a hydrodynamics-based procedure. Gene Ther. 2000, 7, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Liu, D. Hydrodynamic gene delivery: Its principles and applications. Mol. Ther. 2007, 15, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Gao, X.; Stolz, D.B.; Liu, D. Structural impact of hydrodynamic injection on mouse liver. Gene Ther. 2007, 14, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, K.; Liu, D. Physical approaches for nucleic acid delivery to liver. AAPS J. 2008, 10, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, K.; Suda, T.; Zhang, G.; Liu, D. Advances in gene delivery systems. Pharm. Med. 2011, 25, 293–306. [Google Scholar] [CrossRef]
- Bonamassa, B.; Hai, L.; Liu, D. Hydrodynamic gene delivery and its applications in pharmaceutical research. Pharm. Res. 2011, 28, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yao, Q.; Liu, D. Hydrodynamic cell delivery for simultaneous establishment of tumor growth in mouse lung, liver and kidney. Cancer Biol. Ther. 2011, 12, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Wooddell, C.I.; Reppen, T.; Wolff, J.A.; Herweijer, H. Sustained liver-specific transgene expression from the albumin promoter in mice following hydrodynamic plasmid DNA delivery. J. Gene Med. 2008, 10, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Hibbitt, O.C.; Harbottle, R.P.; Waddington, S.N.; Bursill, C.A.; Coutelle, C.; Channon, K.M.; Wade-Martins, R. Delivery and long-term expression of a 135 kb LDLR genomic DNA locus in vivo by hydrodynamic tail vein injection. J. Gene Med. 2007, 9, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Anavi, S.; Hahn-Obercyger, M.; Margalit, R.; Madar, Z.; Tirosh, O. A novel antihypoglycemic role of inducible nitric oxide synthase in liver inflammatory response induced by dietary cholesterol and endotoxemia. Antioxid. Redox Signal. 2013, 19, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- Rydz, N.; Swystun, L.L.; Notley, C.; Paterson, A.D.; Riches, J.J.; Sponagle, K.; Boonyawat, B.; Montgomery, R.R.; James, P.D.; Lillicrap, D. The C-type lectin receptor CLEC4M binds, internalizes, and clears von Willebrand factor and contributes to the variation in plasma von Willebrand factor levels. Blood 2013, 121, 5228–5237. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Zhao, H.; Chen, J.; Wu, W.L.; Wang, H.L.; Jiao, G.J.; Chen, Y.Z. Role of recombinant plasmid pEGFP-N1-IGF-1 transfection in alleviating osteoporosis in ovariectomized rats. J. Mol. Histol. 2013, 44, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, C.; Ma, Y.; Bu, L.; Yan, L.; Liu, D. Hydrodynamic delivery of mIL10 gene protects mice from high-fat diet-induced obesity and glucose intolerance. Mol. Ther. 2013, 10, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, D. Hydrodynamic delivery of adiponectin and adiponectin receptor 2 gene blocks high-fat diet-induced obesity and insulin resistance. Gene Ther. 2013, 20, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wang, Y.; Li, G.; Zhang, L.; Zhang, G.W.; Liao, Y.C.; Hanawa, H.; Zou, J. Effect of hydrodynamics-based delivery of IL-18BP fusion gene on rat experimental autoimmune myocarditis. Clin. Exp. Med. 2014, 14, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Hattori, Y.; Tsukada, H.; Koga, K.; Kajiwara, E.; Kawano, K.; Kobayashi, T.; Kamata, K.; Maitani, Y. Adiponectin gene therapy of streptozotocin-induced diabetic mice using hydrodynamic injection. J. Gene Med. 2007, 11, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Shigekawa, M.; Hikita, H.; Kodama, T.; Shimizu, S.; Li, W.; Uemura, A.; Miyagi, T.; Hosui, A.; Kanto, T.; Hiramatsu, N.; et al. Pancreatic STAT3 protects mice against caerulein-induced pancreatitis via PAP1 induction. Am. J. Pathol. 2012, 181, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.L.; Tsai, C.Y.; Luo, Y.H.; Kuo, C.F.; Lin, W.C.; Chang, Y.T.; Wu, J.J.; Chuang, W.J.; Liu, C.C.; Chao, L.; et al. Kallistatin modulates immune cells and confers anti-inflammatory response to protect mice from group a streptococcal infection. Antimicrob. Agents Chemother. 2013, 57, 5366–5372. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.Y.; Paulmurugan, R.; Nielsen, C.H.; Wang, D.S.; Chow, V.; Robbins, R.C.; Gambhir, S.S. A titratable two-step transcriptional amplification strategy for targeted gene therapy based on ligand-induced intramolecular folding of a mutant human estrogen receptor. Mol. Imaging Biol. 2014, 16, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wooddell, C.I.; Hegge, J.O.; Griffin, J.B.; Huss, T.; Braun, S.; Wolff, J.A. Functional efficacy of dystrophin expression from plasmids delivered to mdx mice by hydrodynamic limb vein injection. Hum. Gene Ther. 2010, 21, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Watcharanurak, K.; Nishikawa, M.; Takahashi, Y.; Kabashima, K.; Takahashi, R.; Takakura, Y. Regulation of immunological balance by sustained interferon-gamma gene transfer for acute phase of atopic dermatitis in mice. Gene Ther. 2013, 20, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Cim, A.; Sawyer, G.J.; Zhang, X.; Su, H.; Collins, L.; Jones, P.; Antoniou, M.; Reynes, J.-P.; Lipps, H.-J.; Fabre, J.W. In vivo studies on non-viral transdifferentiation of liver cells towards pancreatic beta cells. J Endocrinol 2012, 214, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.M.; Wang, W.P. Expression and function of fibroblast growth factor (FGF) 7 during liver regeneration. Cell. Physiol. Biochem. 2011, 27, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, Y.; Li, W.; Wu, Q.; Gao, R. Gene transfer of c-met confers protection against D-galactosamine/lipopolysaccharide-induced acute liver failure. Dig. Dis Sci. 2012, 57, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Shashidharamurthy, R.; Machiah, D.; Bozeman, E.N.; Srivatsan, S.; Patel, J.; Cho, A.; Jacob, J.; Selvaraj, P. Hydrodynamic delivery of plasmid DNA encoding human FcgammaR-Ig dimers blocks immune-complex mediated inflammation in mice. Gene Ther. 2012, 19, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Zhou, Y.; Zhang, H.; Qiu, W.; Chen, L.; Cao, H.; Fang, L.; Wen, P.; Tan, R.; Yang, J. Systemic administration of naked plasmid encoding HGF attenuates puromycin aminonucleoside-induced damage of murine glomerular podocytes. Am. J. Physiol. Renal Physiol. 2011, 301, F784–F792. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.; Molina-Portela, P.; Mott, H.; Carrington, M.; Raper, J. Hydrodynamic gene delivery of baboon trypanosome lytic factor eliminates both animal and human-infective African trypanosomes. Proc. Natl Acad. Sci. USA 2009, 106, 19509–19514. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.; Arfi, A.; Seguin, J.; Gandolphe, C.; Scherman, D. Widespread biochemical correction of murine mucopolysaccharidosis type VII pathology by liver hydrodynamic plasmid delivery. Gene Ther. 2009, 16, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hong, S.W.; Choi, H.S.; Lee, L.Y.; Nam, C.; Rhee, Y.; Chung, U.; Lim, S. Experimental parathyroid hormone gene therapy using OC31 integrase. Endocr. J. 2008, 55, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Okumura, A.; Saito, T.; Otani, I.; Kojima, K.; Yamada, Y.; Ishida-Okawara, A.; Nakazato, K.; Asano, M.; Kanayama, K.; Iwakura, Y.; et al. Suppressive role of leukocyte cell-derived chemotaxin 2 in mouse anti-type II collagen antibody-induced arthritis. Arthritis Rheum. 2008, 58, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.H.; Ye, X.; Thompson, A.R. High-level factor VIII gene expression in vivo achieved by nonviral liver-specific gene therapy vectors. Hum. Gene Ther. 2003, 14, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Delogu, S.; Ho, C.; Lee, S.A.; Gui, B.; Jiang, L.; Ladu, S.; Cigliano, A.; Dombrowski, F.; Evert, M.; et al. Inactivation of Spry2 accelerates AKT-driven hepatocarcinogenesis via activation of MAPK and PKM2 pathways. J. Hepatol. 2012, 57, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.C.; Fioravanti, J.; Duitman, E.H.; Medina-Echeverz, J.; Palazon, A.; Arina, A.; Dubrot, J.; Alfaro, C.; Morales-Kastresana, A.; Murillo, O.; et al. Liver gene transfer of interkeukin-15 constructs that become part of circulating high density lipoproteins for immunotherapy. PLoS ONE 2012, 12, e52370. [Google Scholar] [CrossRef] [PubMed]
- Mukumoto, H.; Takahashi, Y.; Ando, M.; Nishikawa, M.; Takakura, Y. Expression profile-dependent improvement of insulin sensitivity by gene delivery of interleukin-6 in a mouse model of type II diabetes. Mol. Pharm. 2013, 10, 3812–3821. [Google Scholar] [CrossRef] [PubMed]
- Bulau, A.M.; Fink, M.; Maucksch, C.; Kappler, R.; Mayr, D.; Wagner, K.; Bufler, P. In vivo expression of interleukin-37 reduces local and systemic inflammation in concanavalin A-induced hepatitis. Sci. World J. 2011, 11, 2480–2490. [Google Scholar] [CrossRef] [PubMed]
- Zender, L.; Hutker, S.; Liedtke, C.; Tillmann, H.L.; Zender, S.; Mundt, B.; Waltemathe, M.; Gosling, T.; Flemming, P.; Malek, N.P. Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc. Natl Acad. Sci. USA 2003, 100, 7797–7802. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Sun, R.; Wei, H.; Tian, Z. Simultaneous knockdown of multiple ligands of innate receptor NKG2D prevents natural killer cell-mediated fulminant hepatitis in mice. Hepatology 2013, 57, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Marshall, A.L.; Thomas, A.L.; Kernan, K.A.; Su, Y.; LeBoeuf, R.C.; Dong, X.R.; Tchao, B.N. In vivo knockdown of nicotinic acetylcholine receptor alpha1 diminishes aortic atherosclerosis. Atherosclerosis 2011, 215, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shen, H.; Li, A.; Zhang, Z.; Wang, B.; Wang, J.; Zheng, X.; Wu, J.; Yang, D.; Lu, M.; et al. Inhibition of hepatitis B virus (HBV) gene expression and replication by HBx gene silencing in a hydrodynamic injection mouse model with a new clone of HBV genotype B. Virol. J. 2014, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-W.; Zhang, X.-R.; Wang, C.-Z.; Chen, W.-Z.; Xie, W.-F.; Chen, Y.-X. RNA interference targeting the platelet-derived growth factor receptor beta subunit ameliorates experimental hepatic fibrosis in rats. Liver Int. 2008, 28, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, A.P.; Meuse, L.; Pham, T.T.-T.; Conklin, D.S.; Hannon, G.J.; Kay, M.A. Gene expression: RNA interference in adult mice. Nature 2002, 418, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, S.M.; Lo, C.Y.; Tumpey, T.M.; Epstein, S.L. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc. Natl Acad. Sci. USA 2004, 101, 8682–8686. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, H.; Mukoyama, M.; Nagae, T.; Mori, K.; Suganami, T.; Sawai, K.; Yoshioka, T.; Koshikawa, M.; Nishida, T.; Takigawa, M.; et al. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2004, 15, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.D.; Asada, H.; Kishida, T.; Itokawa, Y.; Nakaya, T.; Ueda, Y.; Yamagishi, H.; Goji, S.; Kita, M.; Imanishi, J.; et al. Intravascular naked DNA vaccine encoding glycoprotein B induces protective humoral and cellular immunity against herpes simplex virus type 1 infection in mice. Gene Ther. 2003, 10, 2059–2066. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.L.; Liao, F. Melanoma differentiation-associated gene 5 senses hepatitis B virus and activates innate immune signaling to suppress virus replication. J. Immunol. 2013, 191, 3264–3276. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Yang, W.; Huang, Y.; Wang, Y.; Xiang, L.; Qi, J. Leu452His Mutation in Lipoprotein Lipase Gene Transfer Associated with Hypertriglyceridemia in Mice in vivo. PLoS ONE 2013, 8, e75462. [Google Scholar] [CrossRef] [PubMed]
- Bloom, K.; Ely, A.; Mussolino, C.; Cathomen, T.; Arbuthnot, P. Inactivation of hepatitis B virus replication in cultured cells and in vivo with engineered transcription activator-like effector nucleases. Mol. Ther. 2013, 21, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Shin, D.; Lee, H.; Ahn, B.-Y.; Yoon, Y.; Kim, M. Targeted delivery of siRNA against hepatitis C virus by apolipoprotein A-I-bound cationic liposomes. J. Hepatol. 2009, 50, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Sigal, L.J.; Lerro, A.; Taylor, J. Replication of the human hepatitis delta virus genome is initiated in mouse hepatocytes following intravenous injection of naked DNA or RNA sequences. J. Virol. 2001, 75, 3469–3473. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.L.; Hung, K.C.; Chang, W.T.; Li, E.I.C. Establishment of an early liver fibrosis model by the hydrodynamics-based transfer of TGF-beta1 gene. Comp. Hepatol. 2007, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Tward, A.D.; Jones, K.D.; Yant, S.; Cheung, S.T.; Fan, S.T.; Chen, X.; Kay, M.A.; Wang, R.; Bishop, J.M. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc. Natl Acad. Sci. USA 2007, 104, 14771–14776. [Google Scholar] [CrossRef] [PubMed]
- Wesche-Soldato, D.E.; Lomas-Neira, J.; Perl, M.; Chung, C.-S.; Ayala, A. Hydrodynamic delivery of siRNA in a mouse model of sepsis. Methods Mol. Biol. 2008, 442, 67–73. [Google Scholar] [PubMed]
- Kamimura, K.; Suda, T.; Xu, W.; Zhang, G.; Liu, D. Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Mol. Ther. 2009, 17, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, K.; Guisheng, Z.; Liu, D. Image-guided, intravascular hydrodynamic gene delivery to skeletal muscle in pigs. Mol. Ther. 2010, 18, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, K.; Suda, T.; Zhang, G.; Aoyagi, Y.; Liu, D. Parameters Affecting Image-guided, Hydrodynamic Gene Delivery to Swine Liver. Mol. Ther. Nucleic Acids. 2013, 2, e128. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, K.; Kanefuji, T.; Yokoo, T.; Abe, H.; Suda, T.; Kobayashi, Y.; Zhang, G.; Aoyagi, Y.; Liu, D. Safety assessment of liver-targeted hydrodynamic gene delivery in dogs. PLoS ONE 2014, 24, e107203. [Google Scholar] [CrossRef] [PubMed]
- Eastman, S.J.; Baskin, K.M.; Hodges, B.L.; Chu, Q.; Gates, A.; Dreusicke, R.; Anderson, S.; Scheule, R.K. Development of catheter-based procedures for transducing the isolated rabbit liver with plasmid DNA. Hum. Gene Ther. 2002, 13, 2065–2077. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Hashizume, K.; Kobayashi, E. Naked plasmid DNA transfer to the porcine liver using rapid injection with large volume. Gene Ther. 2006, 13, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Alino, S.F.; Herrero, M.J.; Noguera, I.; Dasi, F.; Sanchez, M. Pig liver gene therapy by noninvasive interventionist catheterism. Gene Ther. 2007, 14, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Fabre, J.W.; Grehan, A.; Whitehorne, M.; Sawyer, G.J.; Dong, X.; Salehi, S.; Eckley, L.; Zhang, X.; Seddon, M.; Shah, A.M. Hydrodynamic gene delivery to the pig liver via an isolated segment of the inferior vena cava. Gene Ther. 2008, 15, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, K.; Suda, T.; Liu, D. Hydrodynamic gene delivery. In Advances and Challenges in the Delivery of Oligonucleotide-Based Therapies; Future Science: London, UK, 2015; In Press. [Google Scholar]
- Suda, T.; Suda, K.; Liu, D. Computer-assisted hydrodynamic gene delivery. Mol. Ther. 2008, 16, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, T.; Kamimura, K.; Suda, T.; Kanefuji, T.; Oda, M.; Zhang, G.; Liu, D.; Aoyagi, Y. Novel electric power-driven hydrodynamic injection system for gene delivery: safety and efficacy of human factor IX delivery in rats. Gene Ther. 2013, 20, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Ginn, S.L.; Alexander, I.E.; Edelstein, M.L.; Abedi, M.R.; Wixon, J. Gene therapy clinical trials worldwide to 2012 - an update. J. Gene Med. 2013, 15, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E. Gene therapy death prompts review of adenovirus vector. Science 1999, 286, 2244–2245. [Google Scholar] [CrossRef] [PubMed]
- Check, E. Second cancer case halts gene-therapy trials. Nature 2003, 421, 305. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamimura, K.; Yokoo, T.; Abe, H.; Kobayashi, Y.; Ogawa, K.; Shinagawa, Y.; Inoue, R.; Terai, S. Image-Guided Hydrodynamic Gene Delivery: Current Status and Future Directions. Pharmaceutics 2015, 7, 213-223. https://doi.org/10.3390/pharmaceutics7030213
Kamimura K, Yokoo T, Abe H, Kobayashi Y, Ogawa K, Shinagawa Y, Inoue R, Terai S. Image-Guided Hydrodynamic Gene Delivery: Current Status and Future Directions. Pharmaceutics. 2015; 7(3):213-223. https://doi.org/10.3390/pharmaceutics7030213
Chicago/Turabian StyleKamimura, Kenya, Takeshi Yokoo, Hiroyuki Abe, Yuji Kobayashi, Kohei Ogawa, Yoko Shinagawa, Ryosuke Inoue, and Shuji Terai. 2015. "Image-Guided Hydrodynamic Gene Delivery: Current Status and Future Directions" Pharmaceutics 7, no. 3: 213-223. https://doi.org/10.3390/pharmaceutics7030213
APA StyleKamimura, K., Yokoo, T., Abe, H., Kobayashi, Y., Ogawa, K., Shinagawa, Y., Inoue, R., & Terai, S. (2015). Image-Guided Hydrodynamic Gene Delivery: Current Status and Future Directions. Pharmaceutics, 7(3), 213-223. https://doi.org/10.3390/pharmaceutics7030213