Analysis of Facial Nerve Functionality and Survival Rates of Patients with Parotid Salivary Gland Carcinoma Submitted to Surgery, Facial Nerve Reconstruction, and Adjuvant Radiotherapy
Abstract
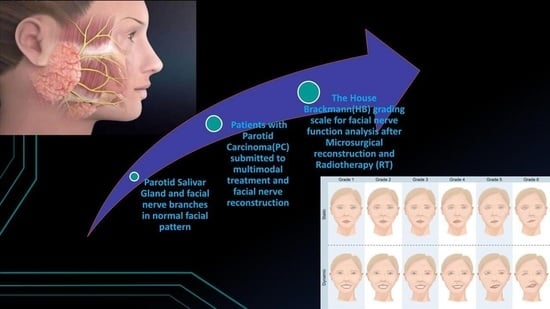
:1. Introduction
2. Materials and Methods
2.1. Population, Samples, and Ethical Approval
2.2. Demographic and Clinical Data/House Brackmann Analysis
2.3. Overall Survival (OS), Disease-Free Survival, and Local Control (LC) Rates
3. Results
Demographic, Clinical, and Treatment Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Mamgani, A.; van Rooij, P.; Verduijn, G.M.; Meeuwis, C.A.; Levendag, P.C. Long-term outcomes and quality of life of 186 patients with primary parotid carcinoma treated with surgery and radiotherapy at the Daniel den Hoed Cancer Center. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, W.; Zhang, C.; Xia, R.; Tian, Z.; Wang, L.; Xia, L.; Li, J. Prognostic nomogram for disease-specific survival of carcinoma ex pleomorphic adenoma of the salivary gland. Head Neck 2017, 39, 2416–2424. [Google Scholar] [CrossRef] [PubMed]
- Osman, B.; Murat, L.; Ahmet, U. Adenoid cystic carcinoma of the parotid gland: Anastamosis of the facial nerve with the great auricular nerve after radical parotidectomy. Indian J. Plast. Surg. 2008, 41, 201–205. [Google Scholar] [CrossRef]
- Villarreal, I.M.; Valiente, R.A.; Castelló, J.R.; Górriz, C.; Montero, O.A.; Berrocal, G.J.R. Promising technique for facial nerve reconstruction in extended parotidectomy. Iran. J. Otorhinolaryngol. 2015, 27, 475–479. [Google Scholar] [PubMed]
- Yu, G.; Peng, X. Conservative and functional surgery in the treatment of salivary gland tumours. Int. J. Oral Sci. 2019, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, M.; Sowa, P.; Sowa, A.M.; Szlęzak, M.; Misiołek, M. Prospective Assessment of Intraoperative Facial Nerve Monitoring in Patients Undergoing Partial Parotidectomy. BioMed Res. Int. 2022, 2022, 3318175. [Google Scholar] [CrossRef] [PubMed]
- Irugu, D.V.K.; Singh, A.; Ch, S.; Panuganti, A.; Acharya, A.; Varma, H.; Thota, R.; Falcioni, M.; Reddy, S. Comparison between early and delayed facial nerve decompression in traumatic facial nerve paralysis—A retrospective study. In CoDAS. SciELO Brasil; SciELO: Sao Paolo, Brazil, 2018; Volume 30, p. e20170063. [Google Scholar]
- Zheng, L.; Tong, L.; Du, F.; Ren, H.; Xiao, L. Effect of three-dimensional conformal radiotherapy and intensity-modulated radiotherapy on parotid gland function and quality of life in patients with nasopharyngeal carcinoma. Am. J. Transl. Res. 2021, 13, 5272–5279. [Google Scholar] [PubMed]
- Gidley, P.W.; Herrera, S.J.; Hanasono, M.M.; Yu, P.; Skoracki, R.; Roberts, D.B.; Weber, R.S. The impact of radiotherapy on facial nerve repair. Laryngoscope 2010, 120, 1985–1989. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhou, S.; Xu, W.; Liu, L.; Lu, H.; Yang, W. Effects of postoperative radiotherapy on vascularized nerve graft for facial nerve repair in a rabbit model. J. Oral Maxillofac. Surg. 2019, 77, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; McGurk, M.; Vaz, F. Management of salivary gland tumours: United Kingdom national multidisciplinary guidelines. J. Laryngol. Otol. 2016, 130, S142–S149. [Google Scholar] [CrossRef] [PubMed]
- Lichius, G.O.; Silver, C.E.; Thielker, J.; Sprekelsen, B.M.; Bradford, C.R.; de Bree, R.; Kowalski, L.P.; Olsen, K.D.; Quer, M.; Rinaldo, A.; et al. Management of the facial nerve in parotid cancer: Preservation or resection and reconstruction. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2615–2626. [Google Scholar] [CrossRef] [PubMed]
- Tanvetyanon, T.; Fisher, K.; Caudell, J.; Otto, K.; Padhya, T.; Trotti, A. Adjuvant chemoradiotherapy versus with radiotherapy alone for locally advanced salivary gland carcinoma among older patients. Head Neck 2016, 38, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Porceddu, S.V.; Bressel, M.; Poulsen, M.G.; Stoneley, A.; Veness, M.J.; Kenny, L.M.; Wratten, C.; Corry, J.; Cooper, S.; Forgarty, G.B.; et al. Postoperative concurrent chemoradiotherapy versus postoperative radiotherapy in high-risk cutaneous squamous cell carcinoma of the head and neck: The randomized phase III TROG 05.01 trial. J. Clin. Oncol. 2018, 36, 1275–1283. [Google Scholar] [CrossRef] [PubMed]




| Demographic, Clinical, and Treatment Data | Number of Patients (n)/Percentage (%) |
|---|---|
| Sex | Male: 49 (53.3%) |
| Female: 43 (46.7%) | |
| Age | ≤60 years: 57 (62%) |
| >60 years: 35 (38%) | |
| Smoking | Smoker: 12 (13%) |
| Non-smoker: 40 (43.5%) | |
| Ex-smoker: 26 (28.3%) | |
| No information: 14 (15.2%) | |
| Alcohol consumption | Alcoholic: 6 (6.5%) |
| Non-alcoholic: 45 (48.9%) | |
| Social alcohol consumption: 12 (13%) | |
| Ex-alcoholic: 7 (7.6%) | |
| No information: 22 (23.9%) | |
| Histological subtype | Squamous-cell carcinoma (SCC): 26 (28.6%) |
| Adenoid cystic carcinoma (ACC): 7 (7.7%) | |
| Mucoepidermoid carcinoma (MC): 10 (11%) | |
| Parotidectomy | Partial: 44 (49.4%) |
| Total: 45 (50.6%) | |
| Microsurgical reconstruction | Yes: 10 (11.1%) |
| No: 80 (88.9%) | |
| Type of nerve flap | Sural nerve: 9 (90%) |
| Other flaps: 1 (10%) | |
| Radiotherapy (RT) | Yes: 73 (79.3%) |
| No: 19 (20.7%) | |
| RT techniques | IMRT: 44 (60.3%) |
| 3D conformal: 23 (31.5%) | |
| Others: 6 (8.2%) | |
| Total dose (Gy) | ≤60 Gy: 41 (57.7%) |
| >60 Gy: 30 (42.3%) | |
| Total sessions (Fx.) | ≤25 fx.: 12 (16.7%) |
| >25 fx.: 60 (83.3%) | |
| Chemotherapy (CT) | Yes: 31 (34.1%) |
| No: 60 (65.9%) | |
| Patient status after treatment | Death for cancer: 23 (25%) |
| Death for other reasons: 5 (5.4%) | |
| Alive with cancer: 4 (4.3%) | |
| Alive without cancer: 47 (51.1%) | |
| Loss of follow up: 13 (14.1%) | |
| Local recurrence (LR) | Local relapse: 24 (26.4%) |
| Non-local relapse: 67 (73.6%) | |
| Locoregional recurrence (LRR) | Locoregional relapse: 8 (9%) |
| Non-locoregional relapse: 81 (91%) | |
| Metastasis | Distant metastasis: 26 (28.3%) |
| Non-distant metastasis: 65 (70.7%) | |
| Local of metastasis | Lung: 20 (21.7%) |
| Bones: 8 (8.7%) | |
| Nervous central system: 6 (6.5%) | |
| Others: 4 (4.3%) |
| Morphological Tumor Criteria | Number of Patients | Percentage |
|---|---|---|
| (n) | (%) | |
| Pathologic staging (pT) | ||
| Tx | 13 | 14.13% |
| T0 | 2 | 2.17% |
| T1 | 19 | 20.65% |
| T2 | 15 | 16.30% |
| T3 | 10 | 10.87% |
| T4 | 6 | 6.52% |
| T4a | 10 | 10.87% |
| T4b | 8 | 8.70% |
| Unknown | 9 | 9.78% |
| Total | 92 | 100% |
| Neck pathologic staging (pN) | ||
| Nx | 14 | 15.22% |
| N0 | 40 | 43.48% |
| N1 | 12 | 13.04% |
| N2 | 2 | 2.17% |
| N2a | 3 | 3.26% |
| N2b | 5 | 5.43% |
| N3 | 3 | 3.26% |
| N3b | 4 | 4.35% |
| Unknown | 9 | 9.78% |
| Total | 92 | 100% |
| Metastasis (pM) | ||
| M0 | 79 | 85.87% |
| M1 | 7 | 7.61% |
| Unknown | 6 | 6.52% |
| Total | 92 | 100% |
| Angiolymphatic invasion | ||
| Yes | 4 | 4.35% |
| No | 86 | 93.48% |
| Unknown | 2 | 2.17% |
| Total | 92 | 100% |
| Vascular invasion (VI) | ||
| Yes | 3 | 3.26% |
| No | 87 | 94.57% |
| Unknown | 2 | 2.17% |
| Total | 92 | 100% |
| Lymph nodes (LNDs) | ||
| Compromised by disease | 23 | 25% |
| Free of disease | 67 | 72.83% |
| Unknown | 2 | 2.17% |
| Total | 92 | 100% |
| Surgical margins | ||
| Compromised by disease | 14 | 15.22% |
| Free of disease | 72 | 78.26% |
| Unknown | 6 | 6.52% |
| Total | 92 | 100% |
| Perineural invasion (PI) | ||
| Yes | 21 | 22.83% |
| No | 69 | 75% |
| Unknown | 2 | 2.17% |
| Total | 92 | 100% |
| Extracapsular extension (ECE) | ||
| Yes | 8 | 8.70% |
| No | 80 | 86.96% |
| Unknown | 4 | 4.35% |
| Total | 92 | 100% |
| Tumor size | ||
| ≤4 cm. | 65 | 70.65% |
| >4 cm. | 21 | 22.83% |
| Unknown | 6 | 6.52% |
| Total | 86 | 100% |
| Number of lymph nodes | ||
| ≤3 | 16 | 69.60% |
| >3 | 7 | 30.40% |
| Total | 23 | 100% |
| HB Grading System | Preoperative Phase N (%) | Postoperative Phase N (%) | Six Months of Follow Up N (%) | One Year N (%) | Two Years N (%) | >2 Years N (%) |
|---|---|---|---|---|---|---|
| I | 68 (87.2%) | 16 (21.1%) | 22 (34.9%) | 27 (48.2%) | 27 (54%) | 28 (57.1%) |
| II | 6 (7.7%) | 20 (26.3%) | 8 (12.7%) | 9 (16.1%) | 7 (14%) | 5 (10.2%) |
| III | 2 (2.6%) | 14 (18.4%) | 11 (17.5%) | 9 (16.1%) | 8 (16%) | 7 (14.3%) |
| IV | 1 (1.3%) | 11 (14.5%) | 10 (15.9%) | 4 (7.1%) | 4 (8%) | 4 (8.2%) |
| V | 1 (1.3%) | 13 (17.1%) | 11 (17.5%) | 6 (10.7%) | 3 (6%) | 4 (8.2%) |
| VI | 0 (0%) | 2 (2.6%) | 1 (1.6%) | 1 (1.8%) | 1 (2%) | 1 (2%) |
| Facial Pattern (FP) | Number of Patients (n) | Percentage (%) |
|---|---|---|
| Normal | 48 | 52.2% |
| Mild facial paresis | 15 | 16.3% |
| Facial nerve palsy | 29 | 31.5% |
| Total | 92 | 100% |
| Radiotherapy | Total | p Value | |||
|---|---|---|---|---|---|
| Yes | No | ||||
| (n) | (n) | (n) | (≤0.05) | ||
| Facial pattern | Normal | 35 (72.9%) | 13 (27.1%) | 48 | 0.28 |
| Mild facial paresis | 13 (86.7%) | 2 (13.3%) | 15 | ||
| Facial nerve palsy | 25 (86.2%) | 4 (13.8%) | 29 | ||
| HB preoperative | Mean ± SD | 1.27 ± 0.76 | 1.06 ± 0.24 | 1.22 ± 0.68 | 0.28 |
| Median/Range | 1.00/1–5 * | 1.00/1–2 * | 1.00/1–5 * | ||
| HB postoperative | Mean ± SD | 2.95 ± 1.54 | 2.65 ± 1.27 | 2.88 ± 1.48 | 0.54 |
| Median/Range | 3.00/1–6 * | 2.00/1–5 * | 3.00/1–6 * | ||
| HB 6 months later | Mean ± SD | 2.88 ± 1.60 | 2.08 ± 1.38 | 2.74 ± 1.58 | 0.12 |
| Median/Range | 3.00/1–6 * | 1.50/1–5 * | 3.00/1–6 * | ||
| HB 1 year later | Mean ± SD | 2.43 ± 1.56 | 1.42 ± 0.67 | 2.21 ± 1.47 | 0.048 |
| Median/Range | 2.00/1–6 * | 1.00/1–3 * | 2.00/1–6 * | ||
| HB 2 years later | Mean ± SD | 2.22 ± 1.46 | 1.22 ± 0.44 | 2.04 ± 1.38 | 0.081 |
| Median/Range | 2.00/1–6 * | 1.00/1–2 * | 1.00/1–6 * | ||
| HB more than 2 years | Mean ± SD | 2.21 ± 1.49 | 1.50 ± 1.27 | 2.06 ± 1.46 | 0.16 |
| Median/Range | 1.00/1–6 * | 1.00/1–5 * | 1.00/1–6 * | ||
| RT Techniques | Total (n) | p Value (≤0.05) | ||||
|---|---|---|---|---|---|---|
| FP | IMRT (n) | 3D (n) | Others (n) | |||
| Facial pattern (FP) | Normal | 19 (54.3%) | 11 (31.4%) | 5 (14.3%) | 35 | 0.24 |
| Mild facial paresis | 11 (84.6%) | 2 (15.4%) | 0 (0%) | 13 | ||
| Facial nerve palsy | 14 (56%) | 10 (40%) | 1 (4%) | 25 | ||
| HB preoperative | Mean ± SD | 1.24 ± 0.63 | 1.39 ± 1.04 | 1.00 ± 0.00 | 0.68 | |
| Median/Range | 1.00/1–4 * | 1.00/1–5 * | 1.00/1 | |||
| HB postoperative | Mean ± SD | 2.95 ±1.45 | 3.11 ±1.68 | 2.25 ± 1.89 | 0.57 | |
| Median/Range | 3.00/1–6 * | 3.00/1–6 * | 1.50/1–5 | |||
| HB 6 months later | Mean ± SD | 3.03 ± 1.49 | 2.83 ± 1.76 | 1.00 ± 0.00 | 0.20 | |
| Median/Range | 3.00/1–5 * | 2.50/1–6 * | 1.00/1 | |||
| HB 1 year later | Mean ± SD | 2.38 ± 1.36 | 2.80 ± 1.88 | 1.00 ± 0.00 | 0.17 | |
| Median/Range | 2.00/1–5 * | 2.00/1–6 * | 2.00/1 | |||
| HB 2 years later | Mean ± SD | 2.08 ± 1.12 | 2.64 ± 1.95 | 1.00 ± 0.00 | 0.34 | |
| Median/Range | 2.00/1–6 * | 1.50/1–6 * | 1.00/1 | |||
| HB more than 2 years | Mean ± SD | 1.96 ± 1.12 | 2.85 ± 1.95 | 1.00 ± 0.00 | 0.22 | |
| Median/Range | 1.50/1–4 * | 3.00/1–6 * | 1.00/1 | |||
| HB Scale Grading/Radiotherapy Performed (RT) | Total Dose Delivered (Gy) | Number of RT Sessions | ||||
|---|---|---|---|---|---|---|
| ρ | p Value | Total | ρ | p Value | Total | |
| HB preoperative | 0.28 | 0.04 | 59 | 0.22 | 0.10 | 60 |
| HB postoperative | 0.09 | 0.50 | 58 | 0.12 | 0.35 | 59 |
| HB 6 months later | 0.13 | 0.37 | 50 | 0.14 | 0.32 | 51 |
| HB 1 year later | 0.29 | 0.06 | 43 | 0.30 | 0.05 | 44 |
| HB 2 years later | 0.24 | 0.13 | 40 | 0.22 | 0.17 | 41 |
| HB more than 2 years later | 0.08 | 0.65 | 38 | 0.03 | 0.86 | 39 |
| Facial Pattern | Total Dose Delivered (Gy) | Number of RT Sessions | ||||
|---|---|---|---|---|---|---|
| Mean | Median | p Value | Mean | Median | p Value | |
| Normal | 57.78 | 60 | 0.72 | 34.12 | 30 | 0.64 |
| Mild facial paresis | 60.38 | 60 | 29.92 | 30 | ||
| Facial nerve palsy | 58.99 | 60 | 28.60 | 31 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernaola-Paredes, W.E.; Novelli, F.; Albuja-Rivadeneira, E.; Flosi, A.A.; Ribeiro, A.V.G.; Nogueira, H.R.; Köhler, H.F.; Pinto, C.A.L.; Vallejo-Rosero, K.A.; Pellizzon, A.C.A. Analysis of Facial Nerve Functionality and Survival Rates of Patients with Parotid Salivary Gland Carcinoma Submitted to Surgery, Facial Nerve Reconstruction, and Adjuvant Radiotherapy. Surg. Tech. Dev. 2023, 12, 68-79. https://doi.org/10.3390/std12020006
Bernaola-Paredes WE, Novelli F, Albuja-Rivadeneira E, Flosi AA, Ribeiro AVG, Nogueira HR, Köhler HF, Pinto CAL, Vallejo-Rosero KA, Pellizzon ACA. Analysis of Facial Nerve Functionality and Survival Rates of Patients with Parotid Salivary Gland Carcinoma Submitted to Surgery, Facial Nerve Reconstruction, and Adjuvant Radiotherapy. Surgical Techniques Development. 2023; 12(2):68-79. https://doi.org/10.3390/std12020006
Chicago/Turabian StyleBernaola-Paredes, Wilber Edison, Franco Novelli, Estefani Albuja-Rivadeneira, Adriana Aparecida Flosi, Anna Victoria Garbelini Ribeiro, Helena Rubini Nogueira, Hugo Fontan Köhler, Clóvis Antonio Lopes Pinto, Kleber Arturo Vallejo-Rosero, and Antonio Cassio Assis Pellizzon. 2023. "Analysis of Facial Nerve Functionality and Survival Rates of Patients with Parotid Salivary Gland Carcinoma Submitted to Surgery, Facial Nerve Reconstruction, and Adjuvant Radiotherapy" Surgical Techniques Development 12, no. 2: 68-79. https://doi.org/10.3390/std12020006
APA StyleBernaola-Paredes, W. E., Novelli, F., Albuja-Rivadeneira, E., Flosi, A. A., Ribeiro, A. V. G., Nogueira, H. R., Köhler, H. F., Pinto, C. A. L., Vallejo-Rosero, K. A., & Pellizzon, A. C. A. (2023). Analysis of Facial Nerve Functionality and Survival Rates of Patients with Parotid Salivary Gland Carcinoma Submitted to Surgery, Facial Nerve Reconstruction, and Adjuvant Radiotherapy. Surgical Techniques Development, 12(2), 68-79. https://doi.org/10.3390/std12020006