Analysis of Biomass Blend Co-Firing for Post Combustion CO2 Capture
Abstract
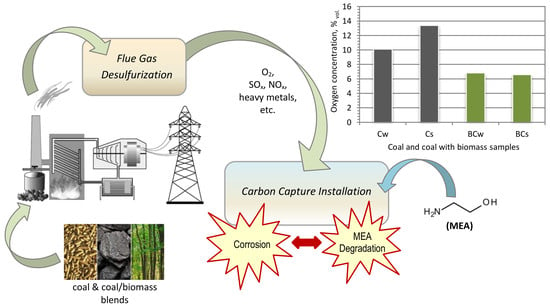
:1. Introduction
2. Oxidative Degradation of MEA Solution
- -
- O2 scavengers and reaction inhibitors, such us hydroquinone, manganese salts, ascorbic acids, Na2SO3, and formaldehyde;
- -
- chelating agents, such as EDTA, sodium phosphate and Na2S4; or
- -
- stable potassium salts, such as KCl, KBr, and formate.
3. Experimental
3.1. Fossil Fuels Characteristics
3.2. Data Analysis
- —substance concentration in flue gas at standard oxygen content in flue gas, mg·Nm3;
- —measured substance concentration in flue gas, mg·Nm3;
- —standard oxygen content in flue gas, vol. % (; and
- —measured oxygen content in flue gas, %.
4. Results
4.1. SO2, NOx and Fly Ash Concentration
4.2. Oxygen Concentration
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The European Parliament; Council of the European Union. Directive 2001/80/EC of 23 October 2001 on the Limitation of Emissions of Certain Pollutiants into the Air from Large Combustion Plants; Council of the European Union: Brussels, Belgium, 2001. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). IPCC Special Report on Carbon Dioxide Capture and Storage: Prepared by Working Group III of the Intergovernmental Panel on Climate Change; Metz, B., Davidson, O., de Coninck, H.C., Loos, M., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2005. [Google Scholar]
- Global CCS Institute. The Global Status of CCS|2013; Global CCS Institute: Melbourne, Australia, 2013. [Google Scholar]
- Baj, S.; Siewniak, A.; Chrobok, A.; Krawczyk, T.; Sobolewski, A. Monoethanolamine and ionic liquid aqueous solutions as effective systems for CO2 capture. J. Chem. Technol. Biotechnol. 2013, 88, 1220–1227. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Khanna, A. CO2 absorption studies in amino acid-anion based ionic liquids. Chem. Eng. J. 2015, 273, 268–276. [Google Scholar] [CrossRef]
- Tobiesen, F.A.; Svendsen, H.F.; Hoff, K.A. Desorber energy consumption amine based absorption plants. Int. J. Green Energy 2005, 2, 201–215. [Google Scholar] [CrossRef]
- Mills, S. Coal-Fired CCS Demonstration Plants, 2012; IEA Clean Coal Centre: London, UK, 2012. [Google Scholar]
- Więcław-Solny, L.; Krótki, A.; Tatarczuk, A.; Stec, M. Operational experiences of different scale Carbon Capture plants. Polityka Energy Energy Policy J. 2014, 17, 393–404. (In Polish) [Google Scholar]
- Krótki, A.; Tatarczuk, A.; Więcław-Solny, L.; Stec, M.; Sobolewski, A.; Tokarski, S. CO2 amine absorption as an oportunity to reduce emission from domestic coal-fired power plants. Przem. Chem. 2014, 12, 2241–2245. [Google Scholar]
- Supap, T.; Saiwan, C.; Idem, R. Part 2: Solvent management: Solvent stability and amine degradation in CO2 capture processes. Carbon Manag. 2011, 2, 551–566. [Google Scholar] [CrossRef]
- Islam, M.N.S.; Yusoff, R.; Ali, B.S.; Chakrabarti, M.H. Degradation studies of amines and alkanolamines during sour gas treatment process. Int. J. Phys. Sci. 2011, 6, 5883–5895. [Google Scholar]
- Irons, R.; Sekkapan, G.; Panesar, R.; Gibbins, J.; Lucquiaud, M. CO2 Capture Ready Plants; Technical Report 2007/4; IEA Greenhouse Gas R&D Programme: Cheltenham, UK, 2007. [Google Scholar]
- Léonard, G.; Voice, A.; Toye, D.; Heyen, G. Influence of dissolved metals and oxidative degradation inhibitors on the oxidative and thermal degradation of monoethanolamine in postcombustion CO2 capture. Ind. Eng. Chem. Res. 2014, 53, 18121–18129. [Google Scholar] [CrossRef]
- Nikolic, H.; Frimpong, R.A.; Liu, K. Trace metals accumulation in a coal-fired post combustion CO2 capture process with amine-based solvents. Int. J. Greenh. Gas Control 2015, 42, 59–65. [Google Scholar] [CrossRef]
- Adams, D. Flue Gas Treatment for CO2 Capture; IEA Clean Coal Centre: London, UK, 2010. [Google Scholar]
- Sexton, A.J.; Rochelle, G.T. Catalysts and inhibitors for oxidative degradation of monoethanolamine. Int. J. Greenh. Gas Control 2009, 3, 704–711. [Google Scholar] [CrossRef]
- De Vroey, S.; Huynh, H.; Lepaumier, H.; Absil, P.; Thielens, M.L. Corrosion investigations in 2-ethanolamine based post-combustion CO2 capture pilot plants. Energy Procedia 2013, 37, 2047–2057. [Google Scholar] [CrossRef]
- Moser, P.; Schmidt, S.; Sieder, G.; Garcia, H.; Stoffregen, T. Performance of MEA in a long-term test at the post-combustion capture pilot plant in Niederaussem. Int. J. Greenh. Gas Control 2011, 5, 620–627. [Google Scholar] [CrossRef]
- Cousins, A.; Ilyushechkin, A.; Pearson, P.; Cottrell, A.; Huang, S.; Feron, P.H.M. Corrosion coupon evaluation under pilot-scale CO2 capture conditions at an Australian coal-fired power station. Greenh. Gases Sci. Technol. 2013, 3, 169–184. [Google Scholar] [CrossRef]
- EBTP-ZEP. Biomass with CO2 Capture and Storage (Bio-CCS) the Way Forward for Europe; JTF Bio-CCS: Rueil-Malmaison, France, 2014. [Google Scholar]
- Kittel, J.; Idem, R.; Gelowitz, D.; Tontiwachwuthikul, P.; Parrain, G.; Bonneau, A. Corrosion in MEA units for CO2 capture: Pilot plant studies. Energy Procedia 2009, 1, 791–797. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Keener, T.C.; Yang, Y.J. Potential flue gas impurities in carbon dioxide streams separated from coal-fired power plants. J. Air Waste Manag. Assoc. 2009, 59, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.M. Post-Combustion Carbon Capture from Coal Fired Plants—Solvent Scrubbing; IEA Clean Coal Centre: London, UK, 2007. [Google Scholar]
- Kittel, J.; Fleury, E.; Vuillemin, B.; Gonzalez, S.; Ropital, F.; Oltra, R. Corrosion in alkanolamine used for acid gas removal: From natural gas processing to CO2 capture. Mater. Corros. 2012, 63, 223–230. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Gas Purification, 5th ed.; Gulf Publishing Company: Houston, TX, USA, 1997. [Google Scholar]
- Krzemień, A.; Więckol-Ryk, A.; Duda, A.; Koteras, A. Risk assessment of a post-combustion and amine-based CO2 Capture Ready Process. J. Sustain. Min. 2013, 12, 18–23. [Google Scholar] [CrossRef]
- Krzemień, A.; Więckol-Ryk, A.; Smoliński, A.; Koteras, A.; Więcław-Solny, L. Journal of loss prevention in the process industries assessing the risk of corrosion in amine-based CO2 capture process. J. Loss Prev. Process Ind. 2016, 43, 189–197. [Google Scholar] [CrossRef]
- Hofmeyer, B.; Scholten, H.G.; Lloyd, W.G. Contamination and corrosion in monoethanolamine gas treating solutions. In Proceedings of the Symposium on Chemicals from Petroleum, Dallas, TX, USA, 8–13 April 1956. [Google Scholar]
- Rao, A.B.; Rubin, E.S. A technical economic and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.; Rochelle, G. Oxidative degradation of monoethanolamine. Ind. Eng. Chem. Res. 2002, 41, 4178–4186. [Google Scholar] [CrossRef]
- Liu, H.; Namjoshi, O.A.; Rochelle, G.T. Oxidative degradation of amine solvents for CO2 capture. Energy Procedia 2014, 63, 1546–1557. [Google Scholar] [CrossRef]
- Supap, T.; Idem, R.; Tontiwachwuthikul, P.; Saiwan, C. Kinetics of sulfur dioxide-and oxygen-induced degradation of aqueous monoethanolamine solution during CO2 absorption from power plant flue gas streams. Int. J. Greenh. Gas Control 2009, 3, 133–142. [Google Scholar] [CrossRef]
- Lepaumier, H.; Da Silva, E.F.; Einbu, A.; Grimstvedt, A.; Knudsen, J.N.; Zahlsen, K.; Svendsen, H.F. Comparison of MEA degradation in pilot-scale with lab-scale experiments. Energy Procedia 2011, 4, 1652–1655. [Google Scholar] [CrossRef]
- Reynolds, A.J.; Verheyen, T.V.; Adeloju, S.B.; Meuleman, E.; Chaffee, A.; Cottrell, A.J.; Feron, P. Chemical characterization of MEA degradation in PCC pilot plants operating in Australia. Energy Procedia 2013, 37, 877–882. [Google Scholar] [CrossRef]
- Goff, G.S.; Rochelle, G.T. Monoethanolamine degradation: O2 mass transfer effects under CO2 capture conditions. Ind. Eng. Chem. Res. 2004, 43, 6400–6408. [Google Scholar] [CrossRef]
- Vevelstad, S.J.; Grimstvedt, A.; Einbu, A.; Knuutila, H.; da Silva, E.F.; Svendsen, H.F. Oxidative degradation of amines using a closed batch system. Int. J. Greenh. Gas Control 2013, 18, 1–14. [Google Scholar] [CrossRef]
- Thong, D.; Dave, N.; Feron, P.; Merched, A. Environmental Impact of Amine-Based CO2 Post Combustion Capture (PCC) Process; Report to ANLECR&D; Commonwealth Scientific and Industrial Research Organisation (CSIRO): Canberra, Australia, 2012. [Google Scholar]
- Goff, G.S.; Rochelle, G.T. Oxidation inhibitors for copper and iron catalyzed degradation of monoethanolamine in CO2 capture processes. Ind. Eng. Chem. Res. 2006, 45, 2513–2521. [Google Scholar] [CrossRef]
- Riemer, P.; Audus, H.; Smith, A. Carbon Dioxide Capture from Power Stations; IEA Greenhouse Gas R&D Programme: Cheltenham, UK, 1993. [Google Scholar]
- Narayanan, K.V.; Natarajan, E. Experimental studies on cofiring of coal and biomass blends in India. Renew. Energy 2007, 32, 2548–2558. [Google Scholar] [CrossRef]
- Smoliński, A.; Howaniec, N.; Stańczyk, K. A comparative experimental study of biomass, lignite and hard coal steam gasification. Renew. Energy 2011, 36, 1836–1842. [Google Scholar] [CrossRef]
- Heinzel, T.; Siegle, V.; Spliethoff, H.; Hein, K.R.G. Investigation of slagging in pulverized fuel co-combustion of biomass and coal at a pilot-scale test facility. Fuel Process. Technol. 1998, 54, 109–125. [Google Scholar] [CrossRef]
- Regulation of the Minister of Environment of 7 November 2014, Concerning Emission Standards for Certain Types of Installations, Fuel Combustion Sources and Waste Firing or Co-Firing Systems. Available online: http://www.dziennikustaw.gov.pl/du/2014/1546/1 (accessed on 19 March 2018).







| Parameter | Sample | |||||
|---|---|---|---|---|---|---|
| Cw | Cs | BCw | BCs | |||
| Coal | Coal | Coal | Biomass | Coal | Biomass | |
| Caloric value (Qia, kJ·kg−1) | 19,765 | 19,665 | 19,705 | 16,551 | 19,395 | 16,245 |
| Ash, Aa (%) | 15.9 | 16.0 | 16.4 | 6.0 | 17.5 | 3.9 |
| Total moisture, Wa (%) | 17.9 | 18.5 | 17.5 | 12.7 | 18.0 | 14.0 |
| Total sulfur Sta (%) | 1.3 | 1.3 | 1.1 | 0.2 | 1.1 | 0.1 |
| Carbon Cta (%) | 52.8 | 51.2 | 52.4 | 45.2 | 50.9 | 44.1 |
| Result | Sample | |||
|---|---|---|---|---|
| Cw | Cs | BCw | BCs | |
| FGD inlet-Block 1 | ||||
| Power (106·kg·m2·s−3) | 179.9 | 182.1 | 161.2 | 144.7 |
| O2 (vol. %) | 5.6 | 6.4 | 6.1 | 6.9 |
| SO2 (mg·Nm−3) | 2810.1 | 2661.0 | 2702.7 | 2389.9 |
| NOx (mg·Nm−3) | 436.8 | 423.3 | 414.9 | 425.3 |
| Fly ash (mg·Nm−3) | 5.6 | 6.4 | 6.1 | 6.9 |
| FGD inlet-Block 2 | ||||
| Power (106·kg·m2·s−3) | 179.3 | 175.9 | 158.9 | 167.3 |
| O2, vol. % | 6.8 | 5.9 | 6.1 | 5.8 |
| SO2 (mg·Nm−3) | 2990.1 | 2914.5 | 2954.9 | 2702.8 |
| NOx (mg·Nm−3) | 196.0 | 188.4 | 195.5 | 180.8 |
| Fly ash (mg·Nm−3) | 15.7 | 8.8 | 8.4 | 10.1 |
| FGD outlet-Absorber | ||||
| O2, vol. % | 10.1 | 13.4 | 6.8 | 6.6 |
| SO2 (mg·Nm−3) | 218.1 | 172.8 | 200.9 | 157.5 |
| NOx (mg·Nm−3) | 365.7 | 302.1 | 338.0 | 286.8 |
| Fly ash (mg·Nm−3) | 4.9 | 4.8 | 2.9 | 3.3 |
| Day | Sample | Day | Sample | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cs | Cw | BCs | BCw | Cs | Cw | BCs | BCw | ||
| 1 | 11.9 | (nd) | 7.3 | 6.8 | 16 | 13.6 | 10.2 | 10.6 | 6.6 |
| 2 | 12.1 | 10.5 | 6.2 | 6.7 | 17 | 13 | 11.1 | 6.4 | 6.7 |
| 3 | 12.0 | 10.0 | 6.3 | 6.7 | 18 | (nd) | 11.3 | 7.25 | 6.2 |
| 4 | 11.8 | 9.2 | 6.1 | 6.6 | 19 | 13.39 | 9.4 | 5.69 | 7.1 |
| 5 | 12.8 | 9.9 | (nd) | 5.7 | 20 | 13.15 | 10.4 | 5.41 | 6.9 |
| 6 | 13.9 | 10.0 | (nd) | 5.9 | 21 | 13.33 | 11.5 | 7.13 | 7.3 |
| 7 | (nd) | 10.6 | 7.4 | 7.7 | 22 | 13.3 | 10.2 | 6.35 | 6.7 |
| 8 | 14.0 | 9.6 | 6.3 | 6.6 | 23 | 13.21 | 9.9 | 6.53 | 7.1 |
| 9 | 14.2 | 9.4 | 5.2 | 6.3 | 24 | 13.41 | 9.4 | 7.05 | 7.6 |
| 10 | 14.1 | 9.9 | 5.7 | 6.3 | 25 | 13.4 | 9.5 | 6.57 | 7.5 |
| 11 | 14.1 | 10.0 | 5.6 | 6.3 | 26 | 13.54 | 9.2 | 6.90 | 7.1 |
| 12 | 14.3 | 9.7 | 5.6 | 6.5 | 27 | 13.73 | 9.4 | 6.01 | 8.1 |
| 13 | 14.4 | 10.2 | 5.5 | 6.9 | 28 | 13.87 | 9.7 | 6.94 | 7.7 |
| 14 | (nd) | 12.2 | 7.4 | 6.3 | 29 | (nd) | 10.1 | 7.55 | 7.6 |
| 15 | 14.2 | 11.3 | 7.2 | 6.3 | 30 | (nd) | 9.8 | 8.04 | 7.0 |
| Day | Sample | Day | Sample | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cs | Cw | BCs | BCw | Cs | Cw | BCs | BCw | ||
| 1 | 397.3 | (nd) | 304.9 | 316.9 | 16 | 163.7 | 343.2 | 120.1 | 309.2 |
| 2 | 387.4 | 311.2 | 374.1 | 324.3 | 17 | 192.7 | 277.7 | 139.9 | 306.9 |
| 3 | 373.9 | 354.0 | 340.4 | 307.7 | 18 | (nd) | 200.4 | 288.5 | 169.9 |
| 4 | 392.4 | 388.0 | 317.5 | 323.2 | 19 | 148.8 | 374.9 | 150.4 | 260.8 |
| 5 | 329.2 | 386.4 | (nd) | 165.2 | 20 | 190.0 | 326.3 | 157.2 | 291.7 |
| 6 | 195.3 | 374.1 | (nd) | 159.4 | 21 | 178.9 | 163.5 | 278.4 | 285.4 |
| 7 | (nd) | 326.1 | 293.0 | 257.0 | 22 | 175.0 | 337.8 | 321.2 | 348.5 |
| 8 | 285.1 | 388.5 | 300.2 | 301.1 | 23 | 190.3 | 339.2 | 313.4 | 291.0 |
| 9 | 323.1 | 372.9 | 181.6 | 311.8 | 24 | 194.5 | 360.1 | 289.1 | 298.1 |
| 10 | 284.0 | 385.3 | 184.5 | 349.6 | 25 | 196.9 | 367.8 | 324.8 | 174.2 |
| 11 | 190.8 | 366.4 | 177.3 | 360.3 | 26 | 197.8 | 385.7 | 279.5 | 180.7 |
| 12 | 189.2 | 368.7 | 191.5 | 335.3 | 27 | 183.0 | 391.6 | 151.6 | 259.0 |
| 13 | 184.4 | 349.6 | 208.6 | 307.5 | 28 | 185.4 | 377.1 | 310.9 | 148.3 |
| 14 | (nd) | 141.5 | 297.4 | 366.2 | 29 | (nd) | 366.4 | 280.2 | 245.9 |
| 15 | 136.2 | 280.3 | 288.4 | 366.4 | 30 | (nd) | 370.4 | 167.7 | 285.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Więckol-Ryk, A.; Krzemień, A.; Smoliński, A.; Lasheras, F.S. Analysis of Biomass Blend Co-Firing for Post Combustion CO2 Capture. Sustainability 2018, 10, 923. https://doi.org/10.3390/su10040923
Więckol-Ryk A, Krzemień A, Smoliński A, Lasheras FS. Analysis of Biomass Blend Co-Firing for Post Combustion CO2 Capture. Sustainability. 2018; 10(4):923. https://doi.org/10.3390/su10040923
Chicago/Turabian StyleWięckol-Ryk, Angelika, Alicja Krzemień, Adam Smoliński, and Fernando Sánchez Lasheras. 2018. "Analysis of Biomass Blend Co-Firing for Post Combustion CO2 Capture" Sustainability 10, no. 4: 923. https://doi.org/10.3390/su10040923
APA StyleWięckol-Ryk, A., Krzemień, A., Smoliński, A., & Lasheras, F. S. (2018). Analysis of Biomass Blend Co-Firing for Post Combustion CO2 Capture. Sustainability, 10(4), 923. https://doi.org/10.3390/su10040923